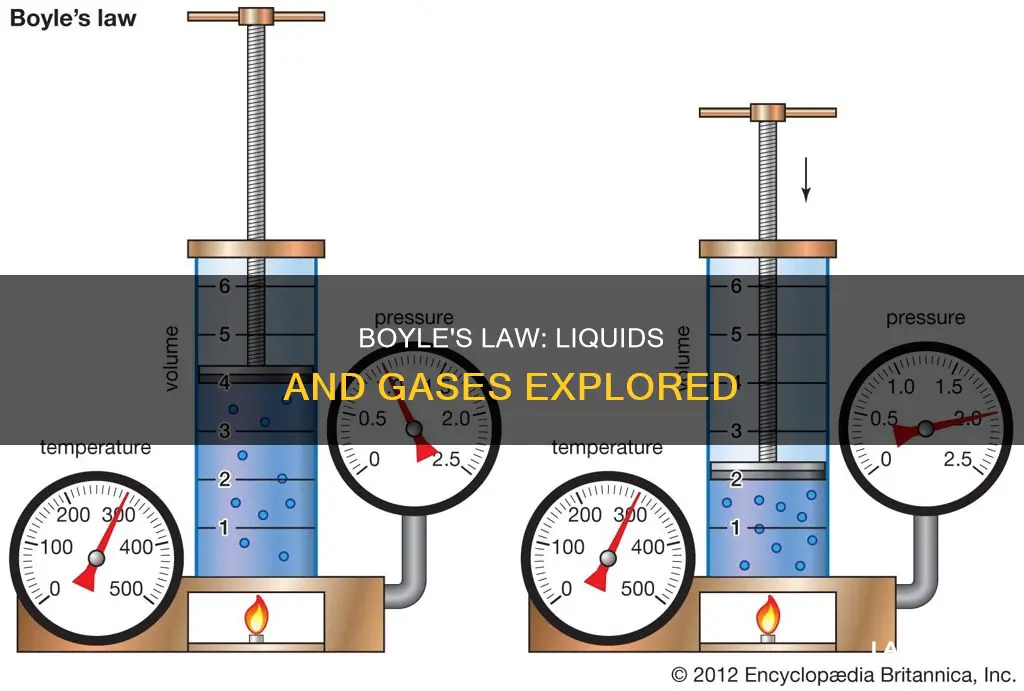
Boyle's Law, also known as Mariotte's Law, is a fundamental law in chemistry that describes the behaviour of a gas held at a constant temperature. The law states that the volume of a gas is inversely proportional to the pressure exerted by the gas. In other words, when the pressure of a gas increases, its volume decreases, and vice versa. This law is based on experiments conducted by Robert Boyle in the 17th century and has been mathematically expressed as pV=k, where p is pressure, V is volume, and k is a constant. While Boyle's Law is applicable to gases, it raises the question of whether it can also be applied to liquids.
| Characteristics | Values |
|---|---|
| Does Boyle's Law apply to liquids? | No, it only applies to gases. |
| What is Boyle's Law? | A basic law in chemistry that describes the behaviour of a gas held at a constant temperature. |
| Who discovered it? | Robert A. Boyle in 1662. |
| What does it state? | The volume of gas is inversely proportional to the pressure exerted by the gas. |
| What is the mathematical representation? | pV=k, where p is pressure, V is volume, and k is a constant. |
| What is an example of Boyle's Law in action? | A balloon. When air is blown into it, the pressure of that air pushes on the rubber, making the balloon expand. |
What You'll Learn

Boyle's Law only applies to gases
Boyle's Law, also known as Mariotte's Law, is a basic law in chemistry that describes the behaviour of a gas held at a constant temperature. The law was discovered by Robert Boyle in 1662 and states that the volume of a gas is inversely proportional to the pressure exerted by the gas. In other words, when a gas is pumped into an enclosed space, its volume decreases, but the pressure that the gas exerts on its container increases.
Boyle's Law can be mathematically written as pV=k, where p is the pressure of the gas, V is the volume of the gas, and k is a constant. This means that when the volume increases, the pressure of a gas decreases proportionally, and vice versa. For example, if the volume is halved, the pressure is doubled, and if the volume is doubled, the pressure is halved.
One example that demonstrates the difference in behaviour between gases and liquids is the activity where a balloon is placed inside a syringe. When the plunger is pushed into the syringe, the air inside the balloon contracts, and the volume decreases. However, when the same experiment is performed with a water-filled balloon, the water inside the balloon does not get compressed, and the volume stays the same. This is because liquids, unlike gases, cannot be compressed as their particles are already very close together.
Copyright Laws: Global Reach of National Legislation?
You may want to see also

Liquids are not compressible
Gases, on the other hand, are highly compressible due to their particles being spread out with plenty of space to move around. This means that when placed under pressure, gas particles can be forced to occupy a smaller volume, resulting in compression.
Boyle's Law, established by Robert Boyle in 1662, specifically addresses the relationship between the volume and pressure of a gas when its temperature remains constant. It states that the pressure exerted by a given mass of an ideal gas is inversely proportional to the volume it occupies, provided that the temperature and amount of gas remain constant. Mathematically, this can be represented as PV = K, where P denotes pressure, V denotes volume, and K is a constant value.
However, this law does not apply to liquids because they are already incompressible. For example, consider a balloon filled with air or water. When pressure is applied to the air-filled balloon, the air inside can escape, causing the balloon to shrink. In contrast, the water inside a balloon is not compressible, and the balloon will remain the same size despite the applied pressure.
Another example of the compressibility of gases is observed when opening a carbonated beverage. The built-up pressure inside the bottle is released, causing the gas-liquid mixture to rush out. This is due to the carbon dioxide gas added to the liquid to create fizz. When the bottle is opened, the available volume for the gas increases, and the pressure decrease, resulting in the gas escaping.
Non-Discrimination Laws: Religious Organizations' Exemptions Explored
You may want to see also

Gases have a variable volume
Boyle's Law, discovered by Robert Boyle in 1662, states that the volume of a gas is inversely proportional to the pressure exerted by the gas, provided the temperature and amount of gas remain constant. This means that as the volume of a gas increases, its pressure decreases, and vice versa.
An example of Boyle's Law in action is the fizzing that occurs when opening a bottle of soda. The bottle is pressurised with carbon dioxide gas to keep it dissolved in the liquid. When the bottle is opened, the pressure inside the bottle is released, causing the gas-liquid mixture to rush out.
Another example is what happens when you inflate your bike tires. When you pump air into a tire, the gas molecules inside the tire get compressed and packed closer together. This increases the pressure of the gas, and it starts to push against the walls of the tire, making it feel tighter and more pressurized.
Boyle's Law also applies to breathing. Inhaling and exhaling cause changes in the volume of our chest cavity, which in turn creates low and high pressure in our lungs, resulting in air being sucked into or expelled from our lungs.
Child Labor Laws: Family Business Exempt?
You may want to see also

Boyle's Law can be used to explain breathing
Boyle's Law, a basic law in chemistry, describes the behaviour of a gas held at a constant temperature. The law was discovered by Robert Boyle in 1662 and states that the volume of gas is inversely proportional to the pressure exerted by the gas. In other words, when a gas is pumped into an enclosed space, it will expand to fill that space, and the pressure the gas exerts on its container will increase.
Breathing occurs when the contraction or relaxation of muscles around the lungs changes the total volume of air within the air passages inside the lungs. When the volume of the lungs changes, the pressure of the air in the lungs changes in accordance with Boyle's Law. If the pressure is greater in the lungs than outside, then air rushes out. If the opposite occurs, then air rushes in.
Boyle's Law also applies when using a medical syringe. When the cylinder on the syringe is empty, it is said to be neutral as there is no air. As one pulls back on the plunger, the volume in the cylinder increases, therefore, by Boyle's Law, the pressure decreases. The liquid is thus drawn into the cylinder to balance the pressure within and outside the syringe.
Curfew Laws: Juvenile-Specific or Universal?
You may want to see also

The relationship between pressure and volume
Boyle's Law, also known as Mariotte's Law, describes the relationship between the pressure and volume of a confined gas. The law states that, at a fixed temperature, the volume of a gas is inversely proportional to the pressure exerted by the gas. In other words, the product of the pressure and volume of a gas is a constant. This can be expressed mathematically as pV=k, where p is the pressure of the gas, V is the volume, and k is a constant.
Boyle's Law was discovered by Robert Boyle in 1662, although the French physicist Edme Mariotte also discovered the relationship independently in 1676 (or 1679, according to another source). The law can be understood by considering what happens when a gas is pumped into an enclosed space: it will shrink to fit that space, but the pressure it exerts on its container will increase.
An example of Boyle's Law in action is blowing air into a balloon. The pressure of the air inside the balloon pushes on the rubber, causing the balloon to expand. If one end of the balloon is squeezed, the volume of air decreases, and the pressure inside increases, causing the rest of the balloon to expand.
Boyle's Law can also be applied to understanding the human breathing system. Inhaling and exhaling involve increasing and decreasing the volume of the chest cavity, which creates low and high pressure in the lungs, causing air to move in and out of the lungs.
It is important to note that Boyle's Law only applies to gases, not liquids or solids. This is because the particles in liquids are already very close together, so the volume of a liquid is not affected by changes in pressure in the same way as a gas.
Fair Housing Laws: Multifamily's Rights and Responsibilities
You may want to see also
Frequently asked questions
No, Boyle's law only applies to gases. Liquids are not compressible as their particles are already very close together.
Boyle's law, also known as the Boyle-Mariotte law, describes the relationship between the pressure and volume of a confined gas when its temperature remains constant.
The equation for Boyle's law is PV=k, where P is the pressure of the gas, V is the volume of the gas, and k is a constant.
Boyle's law was discovered by Robert Boyle in 1662.
A common example of Boyle's law is when you open a fizzy drink bottle. The release of built-up pressure inside causes the gas-liquid mixture to rush out.







