Boyle's Law, also known as the Boyle-Mariotte Law, describes the inverse relationship between the pressure and volume of a fixed amount of gas at a constant temperature. The law was formulated by physicist Robert Boyle in 1662 and states that the pressure of a given quantity of gas varies inversely with its volume at a constant temperature. This means that as the pressure on a gas increases, the volume of the gas decreases, and vice versa. The law can be expressed mathematically as PV = k, where P is pressure, V is volume, and k is a constant. While Boyle's Law holds true for most gases at moderate temperatures and pressures, it is important to note that real gases may show deviations from ideal behaviour at extremely high pressures or very low temperatures.
| Characteristics | Values |
|---|---|
| Description | An empirical gas law that describes the relationship between pressure and volume of a confined gas |
| Formula | P ∝ 1/V |
| Formula | PV = k |
| Formula | P1V1 = P2V2 |
| Applicability | Holds true only if the number of molecules (n) and the temperature (T) are both constant |
| Applicability | Applies to all gases at moderate pressures and temperatures |
What You'll Learn

Boyle's Law and pressure
Boyle's Law, also known as the Boyle-Mariotte Law, describes the inverse relationship between the pressure and volume of a fixed amount of gas at a constant temperature. The law was formulated by physicist Robert Boyle in 1662 and mathematically, it can be stated as:
$$P \propto \frac{1}{V}$$
Where P is the pressure of the gas and V is the volume of the gas. The law states that when the temperature of a given mass of confined gas is constant, the product of its pressure and volume is also constant. This can be expressed as:
$$P_1V_1 = P_2V_2$$
Where P1 and V1 represent the initial pressure and volume, and P2 and V2 represent the values after a change. This equation shows that as volume increases, pressure decreases proportionally, and vice versa.
Boyle's Law applies to a fixed amount of an ideal gas kept at a fixed temperature. It holds true only if the number of molecules (n) and the temperature (T) are both constant. The law was discovered through experiments conducted by Boyle, who used a J-shaped tube partially filled with mercury to trap a small amount of gas or air above the mercury column. The volume of the trapped gas was then measured at atmospheric pressure and constant temperature, and more mercury was added to the open arm to increase the pressure on the gas sample. This process was repeated to measure the resulting volume at increased pressure.
Boyle's Law is one of the three primary gas laws, along with Charles' Law and Avogadro's Law. These three laws combine to form the General Gas Equation and the Ideal Gas Law. The Ideal Gas Law describes the relationship between four gas variables: pressure (P), volume (V), the amount of gas (n), and temperature (T).
Vacation Rentals: Fair Housing Laws and Their Applicability
You may want to see also

Boyle's Law and volume
Boyle's Law, also known as the Boyle-Mariotte Law, describes the inverse relationship between the pressure and volume of a fixed amount of gas at a constant temperature. The law was formulated by physicist Robert Boyle in 1662 and states that the pressure (P) of a given quantity of gas varies inversely with its volume (V) at a constant temperature. This relationship can be expressed mathematically as PV = k, where k is a constant.
Boyle's Law can be used to predict the result of introducing a change in volume and pressure to the initial state of a fixed quantity of gas. The relationship can be expressed as P1V1 = P2V2, where P1 and V1 are the initial pressure and volume values, and P2 and V2 are the values of pressure and volume after the change. This law holds true only if the number of molecules (n) and the temperature (T) remain constant.
The law was discovered through experiments conducted by Boyle, in which he used a J-shaped tube partially filled with mercury to trap a small amount of gas or air above the mercury column. By increasing the pressure on the gas sample and measuring the resulting volume, Boyle was able to determine the quantitative relationship between pressure and volume.
Boyle's Law is particularly relevant in understanding the human breathing system. It is often used to explain how lung volume affects air pressure within the lungs, with higher volume leading to lower air pressure and vice versa. This pressure difference between the air inside the lungs and the environmental air pressure facilitates inhalation and exhalation as air moves from high to low pressure.
In summary, Boyle's Law describes the inverse relationship between pressure and volume for a fixed amount of gas at a constant temperature. This law has practical applications, such as in understanding respiratory processes, and provides valuable insights into the behaviour of gases.
Laws and Regulations for PWC Operators: What You Need Know
You may want to see also

Boyle's Law and temperature
Boyle's Law, also known as the Boyle-Mariotte Law or Mariotte's Law, is an empirical gas law that describes the relationship between pressure and volume in a confined gas. The law states that the absolute pressure exerted by a given mass of an ideal gas is inversely proportional to the volume it occupies, as long as the temperature and amount of gas remain unchanged within a closed system. This relationship can be expressed mathematically as:
{\displaystyle P\propto \frac{1}{V}_
Where P is pressure and V is volume. This means that as volume increases, pressure decreases proportionally, and vice versa.
Boyle's Law specifically applies to situations where temperature remains constant. The law can be used to predict the result of introducing a change in volume and pressure to the initial state of a fixed quantity of gas. The initial and final volumes and pressures of the gas are related by the equation:
{\displaystyle P_{1}V_{1}=P_{2}V_{2}_
Where P1 and V1 represent the original pressure and volume, and P2 and V2 represent the second pressure and volume.
Boyle's Law was formulated by the physicist Robert Boyle in 1662 and states that for a given mass of gas, the pressure times the volume is a constant when the temperature is held constant. This relationship was also discovered by the French physicist Edme Mariotte in 1676.
Most gases behave like ideal gases at moderate pressures and temperatures. At higher pressures and lower temperatures, deviations from ideal gas behaviour become noticeable, and the relationship between pressure and volume can only be accurately described using real gas theory.
Boyle's Law is often used to explain how the breathing system works in the human body. It helps to explain how the volume of the lungs may be increased or decreased, which causes a change in air pressure within them. This pressure difference between the air inside the lungs and the environmental air pressure leads to inhalation or exhalation as air moves from high to low pressure.
Applying Early Decision to Columbia Law: Is It Worth It?
You may want to see also

Boyle's Law and gas particles
Boyle's Law, also referred to as the Boyle-Mariotte Law, describes the relationship between pressure and volume in a confined gas. The law states that, at a constant temperature, the volume of a given mass of a dry gas is inversely proportional to its pressure. In other words, as the volume of a gas increases, its pressure decreases, and vice versa. This relationship can be expressed mathematically as:
PV = k
Where P is pressure, V is volume, and k is a constant value that represents the temperature and amount of gas.
Boyle's Law applies to all gases and is particularly useful for predicting the result of introducing a change in volume and pressure to the initial state of a fixed quantity of gas. The law was formulated by the physicist Robert Boyle in 1662 and can be explained by the behaviour of gas particles.
As the pressure on a gas increases, the gas particles are forced closer together, causing the volume of the gas to decrease. Conversely, when the pressure on a gas decreases, the gas particles are able to move further apart, leading to an increase in volume. This is why weather balloons get larger as they rise through the atmosphere to regions of lower pressure; the volume of the gas inside the balloon increases as the external pressure decreases, until an equilibrium is reached.
Boyle's Law is one of three primary gas laws, alongside Charles' Law and Avogadro's Law. These three laws form the basis of the General Gas Equation and the Ideal Gas Law. The Ideal Gas Law combines the three simple gas laws and can be expressed as:
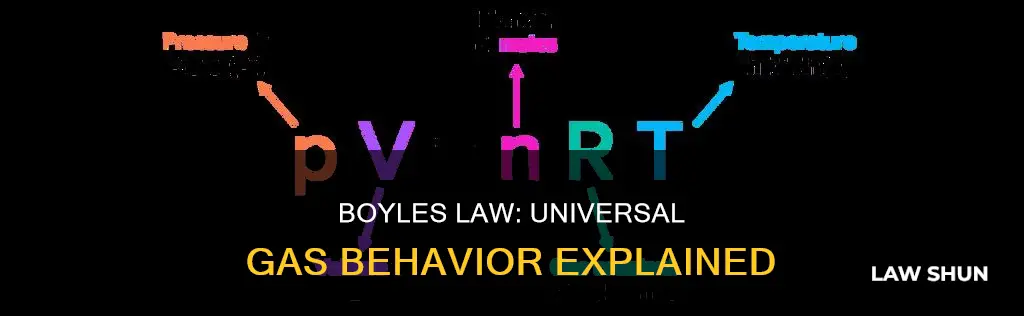
PV = nRT
Where P is the absolute pressure of the ideal gas, V is the volume of the ideal gas, n is the amount of gas, T is the absolute temperature, and R is the gas constant.
Understanding Spring Law: Regular Springs and Ideals
You may want to see also

Boyle's Law and ideal gases
Boyle's Law, also known as the Boyle-Mariotte Law, describes the inverse relationship between the pressure and volume of a fixed amount of gas at a constant temperature. The law is named after chemist and physicist Robert Boyle, who published it in 1662.
The law states that the absolute pressure exerted by a given mass of an ideal gas is inversely proportional to the volume it occupies if the temperature and amount of gas remain unchanged within a closed system. This can be expressed mathematically as:
P ∝ 1/V
Where P is pressure, V is volume, and k is a constant for a particular temperature and amount of gas.
Boyle's Law applies to ideal gases, which are theoretical substances that help establish the relationship between four gas variables: pressure (P), volume (V), the amount of gas (n), and temperature (T). Ideal gases have the following characteristics:
- The particles in the gas are extremely small, so the gas does not occupy any space.
- The ideal gas has constant, random, and straight-line motion.
- There are no forces between the particles of the gas. Particles only collide elastically with each other and with the walls of the container.
Real gases deviate from ideal behaviour at very high pressures and very low temperatures, and the relationship between pressure and volume can only be accurately described using real gas theory. However, most gases behave like ideal gases at moderate pressures and temperatures.
Boyle's Law can be used to predict the result of introducing a change in volume and pressure to the initial state of a fixed quantity of gas. For example, if the volume of a gas is halved, Boyle's Law states that the pressure will double, and if the volume is doubled, the pressure will be halved.
Understanding Texas Escheatment Laws: Who Do They Affect?
You may want to see also







