Graham's Law of Effusion, formulated by Scottish chemist Thomas Graham in 1848, states that the rate of diffusion or effusion of a gas is inversely proportional to the square root of its molar mass. Graham's Law is a gas law that applies to ideal gases at low temperatures and pressures, and it breaks down when the concentration of gases becomes very high. While it is most accurate for molecular effusion, it is only approximate for diffusion. Graham's Law has been crucial in separating isotopes by diffusion, playing a significant role in the development of the atomic bomb.
| Characteristics | Values |
|---|---|
| Name | Graham's Law of Effusion (also called Graham's Law of Diffusion) |
| Formulated by | Scottish physical chemist Thomas Graham |
| Year | 1848 |
| Formula | Rate1/Rate2 = √(M2/M1) |
| Rate1 | Rate of effusion for the first gas (volume or number of moles per unit time) |
| Rate2 | Rate of effusion for the second gas |
| M1 | Molar mass of gas 1 |
| M2 | Molar mass of gas 2 |
| Description | The rate of diffusion or of effusion of a gas is inversely proportional to the square root of its molecular weight |
| Use case | Can be used to find the approximate molecular weight of a gas if one gas is a known species, and if there is a specific ratio between the rates of two gases |
| Application | Graham's law was the basis for separating uranium-235 from uranium-238 during the Manhattan Project to build the first atomic bomb |
What You'll Learn

Graham's Law of Effusion
The law states that the rate of effusion of a gas is inversely proportional to the square root of its molar mass. This means that lighter gases effuse more quickly than heavier gases. This is because, according to the Kinetic Molecular Theory, while two gases at the same temperature have the same average kinetic energy, they do not have the same root mean square speed. Lighter gases will have a higher root mean square speed and will pass through a narrow hole at a faster rate.
Graham's Law can be expressed as:
R_X/(MM_X)^1/2 = r_Y/(MM_Y)^1/2
Where r_X = rate of effusion/diffusion of Gas X, MM_X = molar mass of Gas X, r_Y = rate of effusion/diffusion of Gas Y, and MM_Y = molar mass of Gas Y.
Graham's Law only applies to ideal gases at low temperatures and pressures. As the temperature or pressure increases, the predicted behaviour of the gases deviates from experimental measurements.
Lemon Law: Beyond Cars?
You may want to see also

Diffusion vs Effusion
Graham's Law, formulated by Scottish chemist Thomas Graham in 1848, states that the rate of diffusion or effusion of a gas is inversely proportional to the square root of its molecular weight. This law is most accurate for molecular effusion, which involves the movement of a single gas through a hole. Graham's Law can be applied to both ideal and real gases, although it is only approximate for the diffusion of one gas in another or in the air.
Diffusion and effusion are similar processes involving the movement of gases. Diffusion refers to the movement of gas molecules from regions of high concentration to regions of low concentration. In a closed container, diffusion results in equal concentrations of gas throughout. The rate of diffusion depends on the concentration gradient, the amount of surface area available, and the distance the gas particles must travel.
Effusion, on the other hand, is the escape of gas molecules through a tiny hole, such as a pinhole, into a vacuum. The rate of effusion is influenced by the density and speed of the gas particles. At a given temperature, gases have the same kinetic energy, so their speed is inversely proportional to the square root of their mass. This means that lighter gases have a higher rate of effusion than heavier ones.
While both diffusion and effusion depend on the molar mass of the gas, their rates are not equal. However, the ratios of their rates are the same. The equation for the rate of effusion is:
> rate of effusion ∝ 1/√(molar mass)
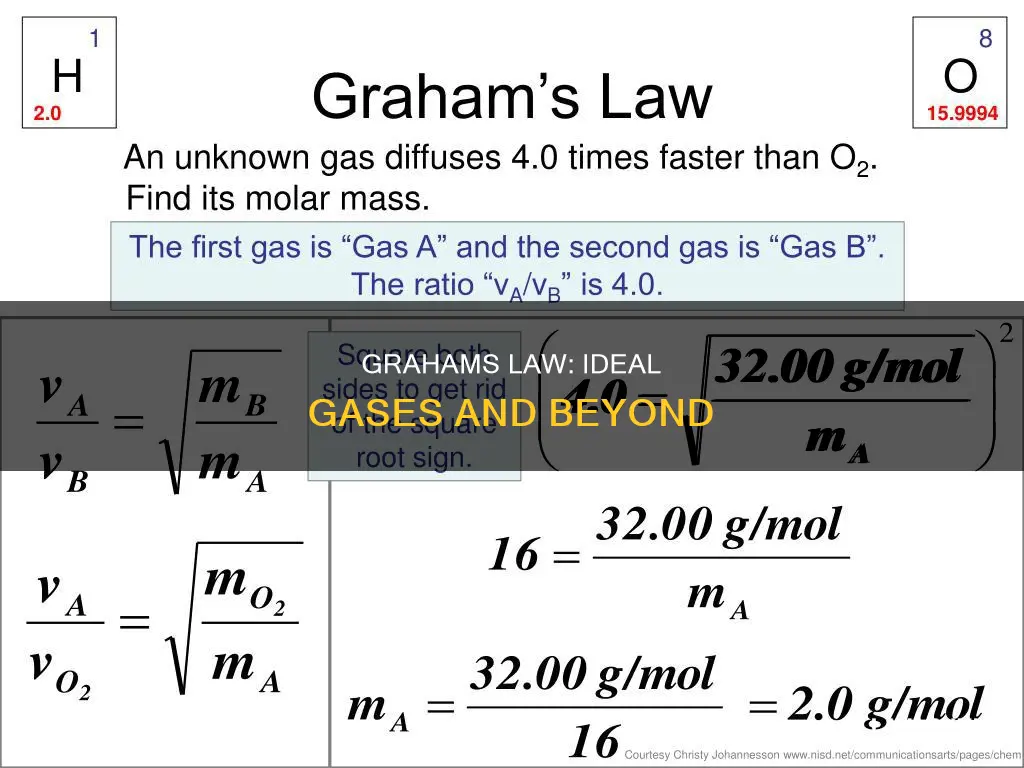
For example, hydrogen effuses four times faster than oxygen.
Backpay Laws: Still Relevant for Current California Employees?
You may want to see also

Molar Mass
Graham's Law of Effusion, formulated by Scottish physical chemist Thomas Graham in 1848, states that the rate of diffusion or effusion of a gas is inversely proportional to the square root of its molar mass. In other words, the rate at which a gas escapes through a small hole is faster for lighter gases and slower for heavier ones.
The distinction between molar mass and molecular mass is important. Molar mass is an average mass of many instances of the compound, taking into account variations in mass due to the presence of isotopes. On the other hand, molecular mass refers to the mass of one specific particle or molecule. The molecular mass of molecules of elements is calculated by multiplying the molar mass of the atoms by the number of atoms in each molecule.
The concept of molar mass is crucial in Graham's Law as it helps establish the relationship between the rate of effusion and the molar mass of the gas. A mathematical representation of Graham's Law is:
> {\displaystyle {{\mbox{Rate}}_{1} \over {\mbox{Rate}}_{2}}={\sqrt {M_{2} \over M_{1}}}}
Where Rate1 and Rate2 are the rates of effusion for two different gases, and M1 and M2 are their respective molar masses.
Adultery Laws in Washington: What You Need to Know
You may want to see also

Kinetic Theory of Gases
The kinetic theory of gases is a classical model that explains the thermodynamic behaviour of gases. It treats a gas as a collection of numerous particles, too small to see with a microscope, that are in constant random motion and frequently collide with each other and the walls of their container. The particles are now known to be the atoms or molecules of the gas. This theory was first introduced by Daniel Bernoulli in 1738, in his work "Hydrodynamica", which laid the basis for the kinetic theory of gases.
The basic version of the model describes an ideal gas. It treats the collisions as perfectly elastic and as the only interaction between the particles, which are assumed to be much smaller than the average distance between them. The gas is assumed to consist of very small particles, with the sum of their volumes being negligible compared to the volume of the container. The number of particles is so large that a statistical treatment of the problem is justified. The rapidly moving particles constantly collide with each other and the walls of the container, and all these collisions are perfectly elastic.
The individual molecules possess the standard physical properties of mass, momentum, and energy. The density of a gas is the sum of the mass of the molecules divided by the volume occupied by the gas. The pressure of a gas is a measure of the linear momentum of the molecules. As the gas molecules collide with the walls of a container, they impart momentum to the walls, producing a force that can be measured. The temperature of a gas is a measure of the mean kinetic energy of the gas. The molecules are in constant random motion, and there is an energy associated with that motion. The higher the temperature, the greater the motion.
The kinetic theory of gases is not just applicable to gases in thermodynamic equilibrium but also to gases not in thermodynamic equilibrium, which are known as "transport properties". This includes viscosity, thermal conductivity, mass diffusivity, and thermal diffusion. The theory has been extended to dense gas mixtures through Revised Enskog Theory, which was developed in 1983-1987.
Graham's Law of effusion, formulated by Scottish physical chemist Thomas Graham in 1848, states that the rate of diffusion or effusion of a gas is inversely proportional to the square root of its molecular weight. This law can be understood as a consequence of the molecular kinetic energies being equal at the same temperature, which is a result of the kinetic theory of gases.
Antitrust Laws: Should NCAA Play by Different Rules?
You may want to see also

Effusion Rate
Effusion is the process of a gas escaping its container through a small opening, such as a pinhole in a vessel or a puncture in a balloon. The rate of effusion is the volume or number of moles of a gas that escapes per unit time.
The rate of effusion is dependent on the molar mass of the gas particles. Graham's Law of Effusion, formulated by Scottish chemist Thomas Graham in 1848, states that the rate of effusion of a gas is inversely proportional to the square root of its molar mass. This can be expressed as:
> {\displaystyle {{\mbox{Rate}}_{1} \over {\mbox{Rate}}_{2}}={\sqrt {M_{2} \over M_{1}}}}
Where Rate1 is the rate of effusion for the first gas, Rate2 is the rate of effusion for the second gas, M1 is the molar mass of gas 1, and M2 is the molar mass of gas 2.
This means that lighter gases with lower molar masses will effuse faster than heavier gases. For example, hydrogen molecules (H2) are 16 times lighter than oxygen molecules (O2) and will therefore effuse out of a container four times faster.
Graham's Law is most accurate for molecular effusion, which involves the movement of one gas at a time through a hole. It is only approximate for the diffusion of one gas into another or into the air, as these processes involve the movement of more than one gas.
Graham's Law is a gas law and was derived from the Kinetic Theory of Gases. It is valid only for ideal gases, which are at low temperatures and pressures. As the temperature or pressure increases, the behaviour of real gases will deviate from the predicted behaviour of ideal gases.
Family Law Statutes: Civil Cases' Application Explored
You may want to see also







