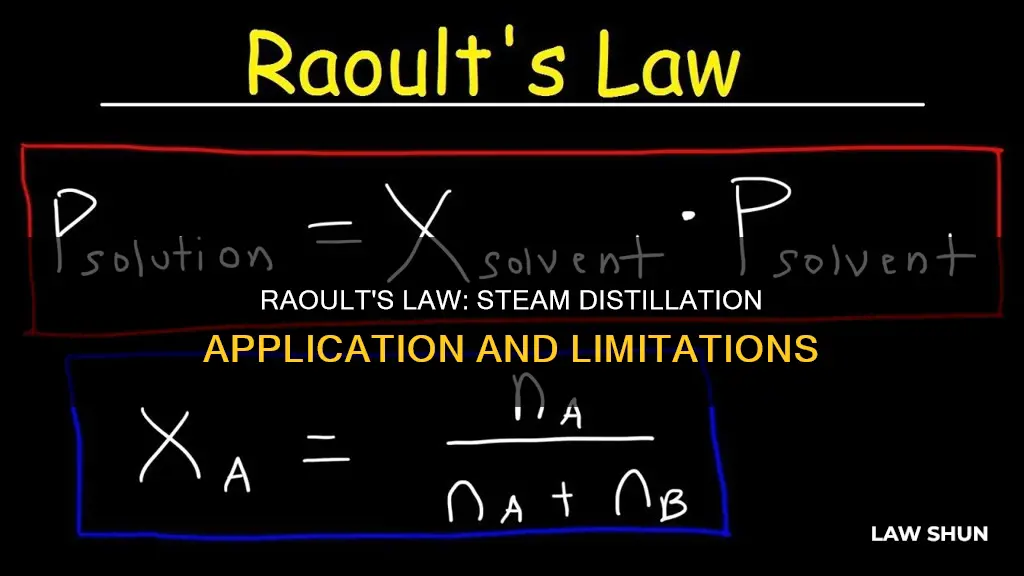
Raoult's law states that a compound's vapour pressure is lessened when it is part of a solution, and is proportional to its molar composition. It is defined as a principle that describes ideal behaviour in a solution by relating the partial vapour pressure of a component to its pure vapour pressure. It is based on the assumption that molecules of all components in the solution are of comparable size.
Raoult's law applies to the distillation of mixtures, which involves boiling a solution and condensing its vapours. However, it does not apply to steam distillation, which involves the distillation of immiscible liquids that do not dissolve in one another. In steam distillation, the components act independently, and the partial pressure of each component is simply its vapour pressure. The vapour composition for a two-component mixture is described by the equation:
> P_solution = P_A^o + P_B^o
This means that the vapour pressures of each component add up, and the mixture will always boil at a lower temperature than the boiling point of the lowest boiling component.
| Characteristics | Values |
|---|---|
| What is Raoult's Law? | A principle that describes ideal behaviour in a solution by relating the partial vapour pressure of a component to its pure vapour pressure. |
| What does Raoult's Law state? | That a compound's vapour pressure is lessened when it is part of a solution, and is proportional to its molar composition. |
| Does Raoult's Law hold for a mixture of immiscible liquids? | No. |
| Why does Raoult's Law not hold for a mixture of immiscible liquids? | The vapour pressure of the solution increases and is equal to the vapour pressures of the two solvents independently. |
| Does Raoult's Law hold for a mixture of immiscible liquids undergoing steam distillation? | No. |
What You'll Learn

Raoult's Law and Dalton's Law
Raoult's Law
Raoult's Law states that the vapour pressure of a solution is dependent on the mole fraction of a solute added to the solution. In other words, it claims that the partial vapour pressure of a compound is lessened when it is part of a solution and is proportional to its molar composition.
Mathematically, Raoult's Law can be expressed as:
> p = P ⋅ x
Where:
- P is the partial pressure of a gas
- P is the pressure of the same gas at the same temperature and volume
- X is the mole fraction
Raoult's Law is particularly relevant in the context of distillation, as it helps understand how the composition of a solution changes during this process. When a solution is heated, the vapour pressure of each component increases, and the component with the higher vapour pressure will have a greater tendency to escape the solution and enter the gas phase.
Dalton's Law
Dalton's Law, on the other hand, pertains to non-reacting gases. It states that the total pressure in a closed system is equal to the sum of the partial pressures of each gaseous component.
Mathematically, Dalton's Law can be written as:
> p_total = p_A + p_B
Where:
- P_total is the total pressure of the mixture
- P_A and p_B are the partial pressures of the individual gases
Combining Raoult's Law and Dalton's Law
When dealing with the distillation of solutions, Raoult's Law and Dalton's Law can be combined to describe the composition of the vapour produced. This is particularly useful when dealing with miscible two-component systems.
The combined law takes into account each component's vapour pressure and quantity (mole fraction) and can be expressed as:
> P_solution = P_A^o ⋅ χ_A + P_B^o ⋅ χ_B
Where:
- P_solution is the vapour pressure of the solution
- P_A^o and P_B^o are the vapour pressures of pure components A and B, respectively
- Χ_A and χ_B are the mole fractions of components A and B in the mixture
Application to Steam Distillation
Now, let's address the specific case of steam distillation. Steam distillation is a technique used to separate immiscible liquids, meaning liquids that do not mix with each other. During steam distillation, a mixture of immiscible liquids is heated, causing the liquids to boil and their vapours to mix. This vapour mixture is then collected and condensed, separating the two liquids.
An important question arises: does Raoult's Law apply to steam distillation? The answer is no, Raoult's Law does not apply directly to steam distillation of immiscible liquids. This is because Raoult's Law assumes that the components in the solution are miscible and form an ideal solution. In an ideal solution, the mole fraction of a component in the vapour phase is proportional to its mole fraction in the liquid phase, as described by Raoult's Law.
However, in the case of immiscible liquids, the total vapour pressure of the mixture is simply the sum of the vapour pressures of the individual liquids. This is because each liquid behaves independently, and their vapour pressures do not depend on their mole fractions in the mixture. Therefore, when dealing with steam distillation of immiscible liquids, Raoult's Law is not applicable, and the behaviour of the system is described by the individual vapour pressures of the liquids involved.
Moore's Law: Still Relevant or an Outdated Concept?
You may want to see also

Ideal Solutions and Raoult's Law
Raoult's Law states that the vapour pressure of a solvent above a solution is equal to the vapour pressure of the pure solvent at the same temperature, scaled by the mole fraction of the solvent present. In the 1880s, François-Marie Raoult discovered that when a substance is dissolved in a solution, the vapour pressure of the solution generally decreases. This decrease depends on two variables: the mole fraction of the amount of dissolved solute present, and the original vapour pressure (pure solvent).
Raoult's Law only works for ideal solutions. An ideal solution shows thermodynamic mixing characteristics identical to those of ideal gas mixtures, except ideal solutions have intermolecular interactions equal to those of the pure components. In reality, the decrease in vapour pressure will be greater than that calculated by Raoult's Law for extremely dilute solutions.
Raoult's Law can be applied to the solubility of a gas in a liquid. This is known as Henry's Law, which states that the mole fraction of a gaseous solute is proportional to the partial pressure of that gas above the solution.
In actual fact, very few liquid mixtures obey Raoult's Law exactly. Even for molecules as similar as benzene and toluene, there is a slight deviation. Much larger deviations occur if the molecules are not very similar. These deviations can be positive or negative. Negative deviations correspond to cases where attractions between unlike molecules are greater than those between like molecules.
Raoult's Law can be applied to steam distillation. Steam distillation is used to separate immiscible liquids rather than solutions. A mixture of immiscible liquids will boil when their combined vapour pressure reaches atmospheric pressure. This combined vapour pressure is the sum of the vapour pressures of each liquid individually, and is independent of the quantities of each phase present.
Raoult's Law is obeyed by a mixture of similar compounds. They are said to form ideal solutions. The substances A and B would form an ideal solution if intermolecular forces between A and B were roughly equal to the intermolecular forces within A and within B. In other words, A sticks to B with roughly equal energy as A sticks to A, and B sticks to B.
Two substances will form an ideal solution when intermolecular forces within each substance and between the two substances are the same. Although there are no ideal gases, ideal solutions may exist if the molecules are almost identical chemically. For example, 1-butanol and 2-butanol form an almost ideal solution.
The highly diluted solution also behaves as an ideal solution due to fewer interactions between solute molecules and solute-solvent molecules.
Charles' Law: Liquids and Their Compressibility
You may want to see also

Raoult's Law and Henry's Law
Raoult's Law
Raoult's law is valid for ideal liquids. The assumption behind Raoult's law is that both the liquid phase and the vapour phase behave ideally. This means that the interaction between any two molecules in the liquid and the vapour have no interaction. The law states that the mole fraction of a component in the liquid phase is proportional to the mole fraction of the same component in the vapour phase. This is only valid for mixtures of ideal liquids.
Raoult's law describes the dependence of the vapour pressure of a solvent as a function of its mole fraction. It is used to calculate the relative lowering of vapour pressure of a solvent when a solute is added.
Henry's Law
Henry's law is also defined for an ideal mixture, with the assumption that the mole fraction of the component tends to zero. This means that when very few molecules are present in a vast space, the interaction between them is almost zero. This makes the system ideal.
Henry's law describes the dependence of the vapour pressure of a solute as a function of its concentration. In terms of mole fraction, Henry's law describes the partial pressure of a component at very low concentration.
Differences
Henry's law constant tends to have huge values. This ensures that even small amounts of solute are accounted for when calculating the mixture properties.
Raoult's law is independent of the nature of the solute; the only parameter is the mole fraction of the solvent (or solute if written as the difference between total pressure and the vapour pressure of the solvent). Henry's law, on the other hand, depends on the nature of both solute and solvent.
Raoult's law can be used to find the vapour pressure of different components of a solution dissolved in it, whereas Henry's law is used to find the amount of solute that can be dissolved in the solution or to find the solubility of the solute.
Curfew Laws: Juvenile-Specific or Universal?
You may want to see also

Raoult's Law and Azeotropes
Raoult's law states that the vapour pressure of a solution is dependent on the mole fraction of a solute added to the solution. It is obeyed by a mixture of similar compounds, and two substances will form an ideal solution when intermolecular forces within each substance and between the two substances are the same.
An azeotrope is a mixture of two or more liquids whose proportions cannot be altered or changed by simple distillation. This is because when an azeotrope is boiled, the vapour has the same proportions of constituents as the unboiled mixture. Azeotropes can be either maximum or minimum boiling, and they occur when the fraction of the liquids cannot be altered by distillation.
Azeotropes exist in solution at a boiling point specific to that component. A maximum-boiling azeotrope is said to be a negative azeotrope because its boiling point is higher than the boiling point of its components. A positive azeotrope, on the other hand, has a lower boiling point than any of its components.
Azeotropes form only when a mixture deviates from Raoult's law. A solution that shows a greater positive deviation from Raoult's law forms a minimum boiling azeotrope at a specific composition, while a solution that shows a large negative deviation forms a maximum boiling azeotrope.
Distillation is one of the primary tools used to separate mixtures, but it cannot separate the constituents of an azeotrope. This is because the separation of azeotropic mixtures, also called azeotrope breaking, is a complex process.
Understanding Moratorium Law: Who Does It Affect?
You may want to see also

Raoult's Law and Steam Distillation
Raoult's law states that the partial vapour pressure of a component in a solution is equal to the vapour pressure of the pure component multiplied by its mole fraction in the mixture. In other words, the law describes the ideal behaviour of a solution by relating the partial vapour pressure of a component to its pure vapour pressure.
Raoult's law applies to solutions of volatile liquids, where each component has a measurable vapour pressure of its own. The law can be applied to a mixture of two or more liquids, where the total vapour pressure of the solution varies in a straight-line manner with the mole fraction composition of the mixture.
Steam distillation is used to separate immiscible liquids rather than solutions. It is often used to separate high-boiling liquids from mixtures containing non-volatile impurities. In steam distillation, water is added to a mixture, and the water-oil mixture is heated to 100°C, guaranteeing that the mixture will boil. The water-oil mixture is then separated using a separatory funnel.
Raoult's law can be applied to steam distillation when the components in the distilling flask are miscible. When the components are immiscible, they act independently, and the partial pressure of each component is simply its vapour pressure.
Lemon Law and Leases: What's the Verdict?
You may want to see also
Frequently asked questions
Raoult's Law states that the partial vapour pressure of each component in a mixture is equal to the vapour pressure of the pure component multiplied by its mole fraction in the mixture.
Steam distillation is used to separate immiscible liquids. It involves adding steam to a mixture of two liquids that do not dissolve in each other. The steam helps transport the more volatile component into the vapour phase.
No, Raoult's Law does not apply to steam distillation. In steam distillation, the components in the mixture act independently of each other, and the partial pressure of each component is simply its vapour pressure. The vapour composition for a two-component mixture is described by the equation:
> P_solution = P_A^o + P_B^o
Raoult's Law assumes that the components in the mixture are miscible and that the vapour pressure of the solution is dependent on the mole fraction of the solute added to the solution. In steam distillation, the components are immiscible and the vapour pressure of each component is independent of its mole fraction.
Steam distillation is widely used in industries such as petroleum refining and in the flavours and perfumes industry for the isolation of essential oils. It was invented in the 13th century by Ibn al-Baiter, a physician and botanist in Andalusia.







