Solids and liquids are not included in rate law equations because their concentrations are assumed to be constant. For example, if a reaction takes place in water, the water is in such excess that its concentration remains essentially unchanged. Similarly, only the portion of a solid that dissolves is available to react, and as soon as it reacts, more of the solid dissolves to maintain equilibrium.
| Characteristics | Values |
|---|---|
| --- | --- |
| Rate Law Expression | Does not include solids and liquids as their concentrations are assumed to be constant |
| Solids and Liquids | Included in the rate law expression if they are reactants |
What You'll Learn
- Solids and liquids are not included in rate law equations because their concentrations are assumed to be constant
- For a reaction in a liquid, the rate law does not change
- The rate law for a reaction involving a solid depends on the surface area of the solid
- The rate law for a reaction involving a solid can be determined experimentally
- The rate law for a reaction involving a solid is not typically addressed in introductory chemistry courses

Solids and liquids are not included in rate law equations because their concentrations are assumed to be constant
Solids and liquids are generally not included in rate law equations because their concentrations are assumed to be constant. This assumption is based on the fact that the concentration of a pure liquid or solid cannot be increased, and hence, their concentrations remain relatively constant throughout the reaction.
For instance, in a reaction where water is a reactant and is present in excess, its concentration does not significantly change, and thus, it is not included in the rate law equation. Similarly, for solids, only the portion that dissolves is available to react, and as it reacts, more of the solid dissolves to maintain equilibrium, resulting in a relatively constant concentration.
However, it is important to note that if water is in the gaseous state, its concentration can be included in the rate law equation as it is no longer a pure liquid. Additionally, the concentration of solids and liquids may be included in some equilibrium constant expressions, especially if water is a reactant and not a solvent.
The exclusion of solids and liquids from rate law equations simplifies the mathematical expressions and calculations involved in determining reaction rates. By focusing on the reactants and their concentrations, we can gain valuable insights into the kinetics and mechanisms of reactions.
Maritime Law: Does It Govern Our Lakes?
You may want to see also

For a reaction in a liquid, the rate law does not change
For example, if a reaction takes place in water and water is part of the reaction, the water is in such excess that the concentration essentially does not change. Similarly, for a solid, only the portion that dissolves is available to react, and as soon as it reacts, more of the solid dissolves to maintain equilibrium, keeping the concentration constant.
Moore's Law: The Future of Computing Power?
You may want to see also

The rate law for a reaction involving a solid depends on the surface area of the solid
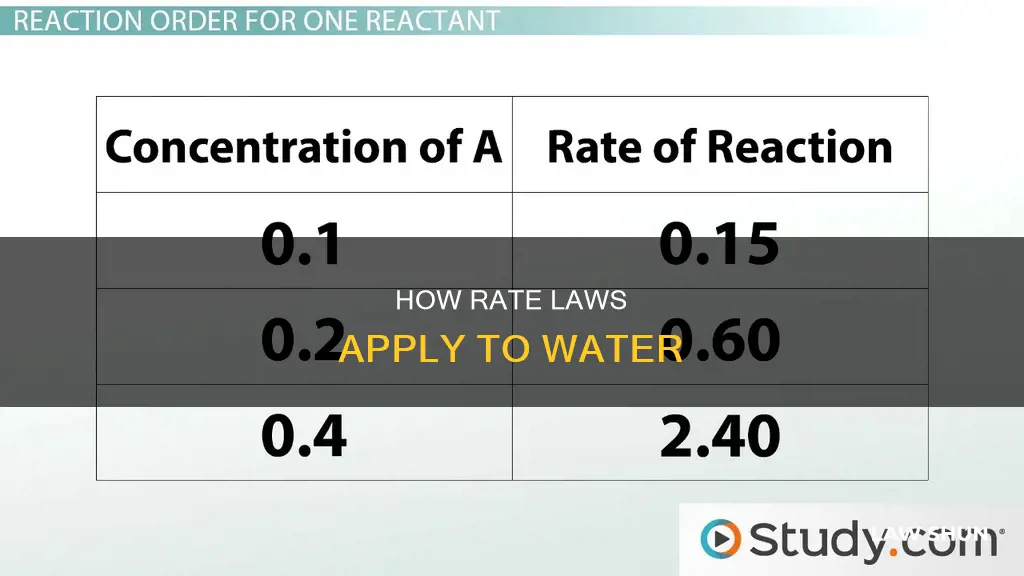
The rate of a reaction is defined as the change in concentration of a substance over the time interval during which this change is observed. In other words, it is the measure of the change in the concentration of the disappearance of reactants or the change in the concentration of the appearance of products per unit time.
The rate law for a given chemical reaction is the measure of the change in concentration of the reactants or the products per unit time. The rate of a reaction can be expressed by any one of the reactants or products in the reaction. The rate law is an expression that relates the rate of a reaction to the rate constant and the concentrations of the reactants.
The rate of a reaction involving a solid depends on the surface area of the solid. This is because the two types of molecules can only collide with each other at the liquid-solid interface, i.e. on the surface of the solid. Therefore, the larger the surface area of the solid, the faster the reaction will be.
For example, if you have a loaf of bread, it has six sides of surface area. If you slice it in half, you will have 12 sides of surface area. If you keep cutting the bread, you will keep increasing the surface area and provide more locations for a reaction to take place.
The rate law for a reaction involving a solid can be expressed as:
> Rate = k[solid]^m
Where k is the rate constant, and m is the reaction order with respect to the solid. The reaction order indicates the degree to which the reaction rate depends on the concentration of the solid.
HOA Communities and Their Strata Title Laws
You may want to see also

The rate law for a reaction involving a solid can be determined experimentally
The rate law for a reaction involving a solid can be determined by performing experiments that measure the concentration of the solid reactant and the rate of the reaction under different conditions. The concentration of the solid reactant can be varied while keeping other factors constant, and the rate of the reaction can be measured to determine the relationship between the two.
For example, let's consider a reaction where solid A reacts with a liquid B to form a product C. The rate of the reaction can be measured at different initial concentrations of solid A while keeping the concentration of liquid B constant. By comparing the rates of the reaction at different concentrations of A, the relationship between the rate and the concentration of A can be determined.
The rate law for this reaction can then be expressed as:
Rate = k [A]^x [B]^y
Where k is the rate constant, x and y are the reaction orders, and [A] and [B] are the concentrations of the reactants. The reaction orders, x and y, can be determined experimentally by measuring the rate of the reaction at different concentrations of A and B.
It is important to note that solids and liquids are typically not included in rate law equations because their concentrations are assumed to be unchanging. For example, if the reaction takes place in water, the water is in such excess that its concentration remains essentially constant. Similarly, for a solid reactant, only the portion that dissolves is available to react, and more solid dissolves as it reacts to maintain equilibrium.
Lemon Law in NJ: Private Sales Included?
You may want to see also

The rate law for a reaction involving a solid is not typically addressed in introductory chemistry courses
The rate law for a chemical reaction is an expression that describes the relationship between the rate of the reaction and the concentrations of the reactants. The rate of a chemical reaction depends on several factors, including the nature of the reactants, surface area, temperature, concentration, and catalysts.
The rate law equation is typically written in the standard form:
> Rate = k [A]^m [B]^n
Where:
- Rate is the reaction rate, expressed in concentration per unit of time (usually molarity per second).
- K is the specific rate constant.
- [A] and [B] are the molar concentrations of reactants A and B, expressed in moles of solute per liter of solution.
- M and n are the orders of the reaction.
Similarly, for a solid, only the portion that dissolves is available to react. As soon as it reacts, more of the solid dissolves to maintain equilibrium, and the concentration remains the same. This simplification is often used in introductory chemistry courses, but in reality, the rate law for reactions involving solids can be more complex.
In summary, while the rate law is a fundamental concept in chemistry, the treatment of reactions involving solids may be simplified or omitted in introductory courses due to their added complexity.
Anti-Discrimination Law: Does It Protect White People?
You may want to see also
Frequently asked questions
No, rate laws do not apply to solids and liquids because their concentrations are assumed to be constant. For example, if a reaction takes place in water, the water is in such excess that its concentration does not change significantly.
Solids and liquids are not included in rate law expressions because their concentrations are effectively constant.
The rate law expression for a reaction involving solids or liquids can be determined experimentally by measuring the rate of the reaction at different concentrations of reactants and then using the data to determine the reaction orders and rate constant.
The presence of solids and liquids does not typically have a significant effect on the rate of a chemical reaction.







