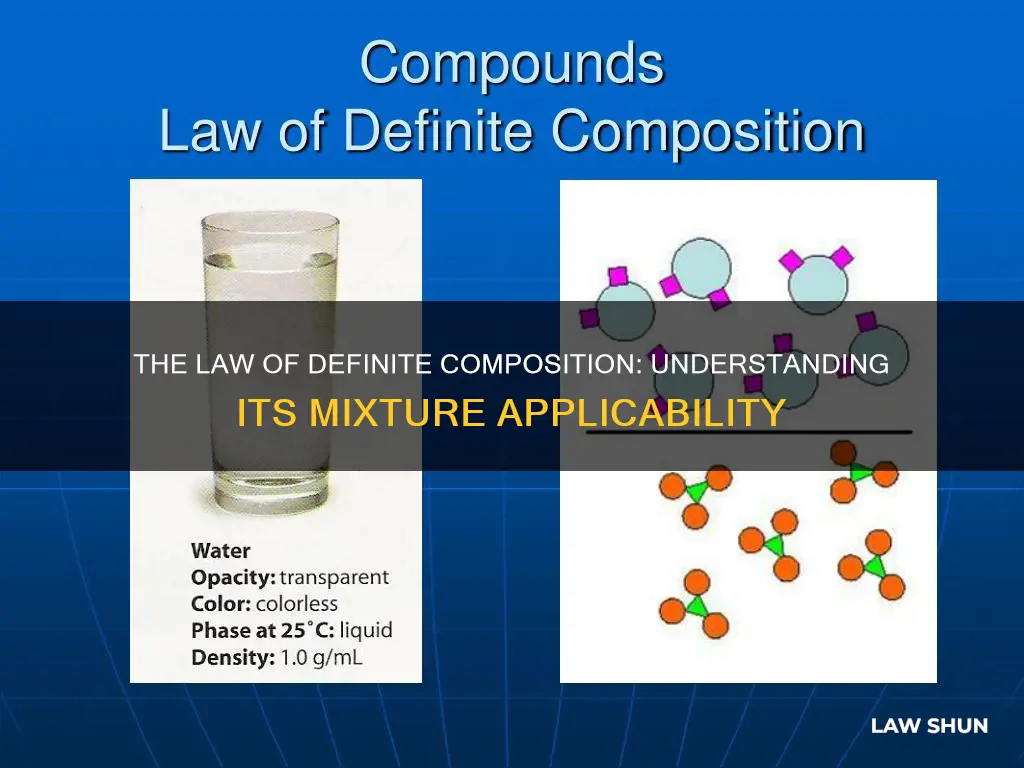
The law of definite composition, also known as Proust's Law, states that a chemical compound always contains its constituent elements in a fixed ratio by mass. This means that the composition of a compound is independent of its source or method of preparation. For example, in any sample of pure water, oxygen makes up about 8/9 of the mass, while hydrogen makes up the remaining 1/9. However, this law does not apply to elements or mixtures. An element contains only one type of atom, so the law does not apply to it. On the other hand, mixtures are not pure chemical compounds, and thus the law of definite composition does not apply to them.
| Characteristics | Values |
|---|---|
| Name | Law of Definite Proportions, Proust's Law, Law of Constant Composition |
| Definition | A chemical compound always contains its component elements in a fixed ratio (by mass) and does not depend on its source or method of preparation |
| Applicability | Only to compounds, not elements |
| Exceptions | Non-stoichiometric compounds, commonly metals |
What You'll Learn
- The law of definite proportions applies only to compounds
- A compound is a substance formed when two or more elements are chemically bonded
- The law states that a compound always contains the same proportion of elements by mass
- The composition of a compound does not depend on its source or method of preparation
- The law of constant composition is the same as the law of definite proportions

The law of definite proportions applies only to compounds
The law of definite proportions, also known as Proust's Law or the law of constant composition, applies only to compounds. It states that a given chemical compound will always contain its constituent elements in a fixed ratio by mass, regardless of its source or method of preparation.
For example, in any sample of pure water, oxygen makes up about 8/9 of the mass, while hydrogen makes up the remaining 1/9. This ratio remains constant across all samples of water. This law forms the basis of stoichiometry, along with the law of multiple proportions.
It is important to note that this law does not apply to elements, as they contain only one type of atom. It also does not apply to all compounds; non-stoichiometric compounds, which are often metals, can have varying elemental compositions from sample to sample. For instance, the iron oxide wüstite can contain between 0.83 and 0.95 iron atoms for every oxygen atom, resulting in a variable mass composition.
The law of definite proportions was first observed by French chemist Joseph Proust in 1794, although the English theologian and chemist Joseph Priestley and French nobleman and chemist Antoine Lavoisier had made similar observations earlier during their studies of combustion. When first proposed, the law was considered controversial, with Proust's compatriot Claude Louis Berthollet arguing that elements could combine in any proportion. However, John Dalton's atomic theory, introduced in 1803, provided a firm theoretical basis for the law, explaining that matter consists of discrete atoms, with compounds formed by combining different types of atoms in fixed proportions.
Leash Laws and Private Property: Understanding the Legal Boundaries
You may want to see also

A compound is a substance formed when two or more elements are chemically bonded
The Law of Definite Proportions, also known as Proust's Law or the Law of Constant Composition, was first observed by French chemist Joseph Proust in 1794. This law applies only to compounds, which are substances formed when two or more elements are chemically bonded together.
The law states that a chemical compound always contains its component elements in a fixed ratio by mass, and this ratio does not depend on the compound's source or method of preparation. For example, in any sample of pure water, oxygen makes up about 8/9 of the mass, while hydrogen makes up the remaining 1/9. This ratio is always the same, no matter the source of the water or how it was prepared.
The law of definite proportions forms the basis of stoichiometry, along with the law of multiple proportions. However, it is important to note that there are some exceptions to this law, known as non-stoichiometric compounds, which are usually metals. These compounds do not always follow the fixed ratio of elements and can vary from sample to sample.
The discovery of the law of definite proportions was significant in the development of chemistry, as it helped establish the concept of a chemical compound and contributed to the atomic theory proposed by John Dalton in 1803. This theory explained that matter consists of discrete atoms, with one type of atom for each element, and that compounds are formed by combining different types of atoms in fixed proportions.
HIPAA Laws: Do They Extend to Military Personnel?
You may want to see also

The law states that a compound always contains the same proportion of elements by mass
The law of definite composition, also known as Proust's Law, was first observed by French chemist Joseph Proust in 1794. The law states that a compound always contains the same proportion of elements by mass. This means that the composition of a compound is fixed and does not depend on its source or method of preparation. For example, in any sample of pure water, oxygen makes up about 8/9 of the mass, while hydrogen makes up the remaining 1/9. This ratio of oxygen to hydrogen remains constant regardless of the source of the water or how it was prepared.
The law of definite composition is a fundamental concept in chemistry, forming the basis of stoichiometry, which is the study of the quantitative relationships between reactants and products in chemical reactions. It is important to note that this law only applies to compounds, which are substances formed when two or more elements are chemically bonded together. An element, on the other hand, contains only one type of atom, so the law of definite composition does not apply to it.
While the law of definite composition is a useful principle in chemistry, it is not universally true. There are some compounds, known as non-stoichiometric compounds, to which this law does not apply. These compounds, which are often metals, can have varying elemental compositions from sample to sample. For example, the iron oxide wüstite can contain between 0.83 and 0.95 iron atoms for every oxygen atom, resulting in a range of 23% to 25% oxygen by mass.
Additionally, it is worth mentioning that the law of definite composition was not always widely accepted. When first proposed by Proust in the late 18th century, it was a controversial statement. Proust's fellow Frenchman, Claude Louis Berthollet, argued that elements could combine in any proportion. This debate highlights that, at the time, the distinction between pure chemical compounds and mixtures was not yet fully understood.
The Law of Conservation: Universal or Not?
You may want to see also

The composition of a compound does not depend on its source or method of preparation
The law of definite proportions, also known as Proust's law or the law of constant composition, states that a chemical compound always contains its component elements in a fixed ratio (by mass) and does not depend on its source or method of preparation. This means that the composition of a compound is not influenced by how or where it was prepared. For example, in any sample of pure water, oxygen always constitutes about 8/9 of the mass, while hydrogen makes up the remaining 1/9 of the mass. This ratio remains constant regardless of the water's origin or the process used to obtain it.
Joseph Proust, a French chemist, first observed this law in 1794, although it was initially proposed by English theologian and chemist Joseph Priestley and French nobleman and chemist Antoine Lavoisier through their studies of combustion. At the time, the concept of a chemical compound was not yet fully developed, and Proust's assertion was considered controversial by some of his contemporaries, such as fellow Frenchman Claude Louis Berthollet, who argued that elements could combine in any proportion.
The law of definite proportions played a crucial role in the development of modern chemistry. It contributed to John Dalton's atomic theory, which explained matter as consisting of discrete atoms, with each element having a unique type of atom, and compounds being formed by the combination of different types of atoms in fixed proportions. This law also forms the basis of stoichiometry, along with the law of multiple proportions.
It is important to note that the law of definite proportions does have some exceptions. There exist non-stoichiometric compounds, typically metals, whose elemental composition can vary from sample to sample. Additionally, the isotopic composition of an element can vary depending on its source, which can lead to slight variations in the mass contribution to a pure stoichiometric compound.
Thermodynamics Laws: Universal or Not?
You may want to see also

The law of constant composition is the same as the law of definite proportions
The law of constant composition, also known as Proust's Law, states that a chemical compound always contains its component elements in a fixed ratio by mass. This means that the compound's composition does not depend on its source or method of preparation. For example, in water, oxygen always makes up about 8/9 of the mass, while hydrogen makes up the remaining 1/9.
To illustrate this, consider two samples of a compound containing hydrogen and oxygen. In the first sample, there is 15.0g of hydrogen and 120.0g of oxygen. In the second sample, there is 2.00g of hydrogen and 32.0g of oxygen. To determine if these samples are the same compound, we can calculate the mass ratio of the elements in each sample. For the first sample, the mass ratio of oxygen to hydrogen is 8:1 (120/15 = 8). For the second sample, the mass ratio is also 8:1 (32/2 = 16, which simplifies to 8:1). Since the mass ratios are the same, we can conclude that the two samples are the same compound.
The law of definite proportions was first proposed by French chemist Joseph Proust in 1794 and later in 1797, although the original observations were made by English theologian and chemist Joseph Priestley, and French nobleman and chemist Antoine Lavoisier. When it was first proposed, the law was controversial and opposed by other chemists, as the distinction between pure chemical compounds and mixtures was not yet fully understood. However, it later contributed to and was supported by John Dalton's atomic theory, which explained that matter consists of discrete atoms and that compounds are made of different types of atoms in fixed proportions.
Arizona's Anti-Bullying Law: Does It Cover Cyberbullying?
You may want to see also
Frequently asked questions
No, the law of definite proportions applies only to compounds.
The law of definite composition, also known as Proust's Law, states that a chemical compound always contains its component elements in a fixed ratio (by mass) and that this ratio does not depend on its source or method of preparation.
A compound is a substance formed when two or more elements are chemically bonded together.
In water, oxygen makes up about 8/9 of the mass, while hydrogen makes up the remaining 1/9, regardless of the source of the water or how it was prepared.
Yes, there are some compounds, typically metals, called non-stoichiometric compounds, where this law does not apply.







