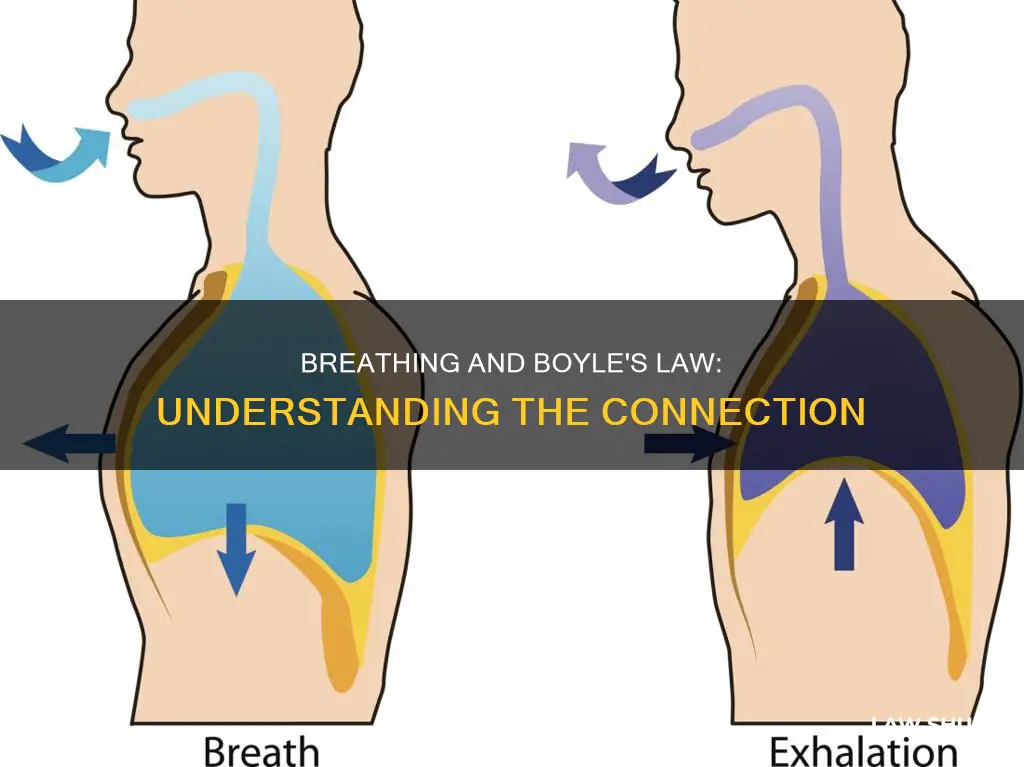
Boyle's law, discovered by Robert Boyle in 1662, states that the volume of a gas and pressure are inversely proportional to each other at a given temperature. This law is integral to understanding the mechanics of breathing. When the lungs expand, the volume inside them increases, and the pressure decreases, allowing air to move into the lungs from outside the body. This process is known as inhalation. During exhalation, the volume inside the lungs decreases, and the pressure increases, forcing air out.
| Characteristics | Values |
|---|---|
| Relationship | Describes the relationship between the pressure and volume of gas for a mass and temperature |
| Formula | PV = K (P is pressure, V is volume, K is a constant) |
| Inverse relationship | Pressure is inversely proportional to volume |
| Application to breathing | As the lungs expand, the volume inside the lungs increases and the pressure inside decreases, causing air to move into the lungs |
| Application to breathing | As the volume inside the lungs decreases, the pressure increases and forces air out |
| Application to syringes | When the plunger is pulled out, the volume inside the syringe increases and the pressure decreases, causing fluid to be drawn into the syringe |
| Application to syringes | When the plunger is pushed in, the volume decreases and the pressure increases, causing fluid to be forced out of the syringe |
| Application to SCUBA diving | As a diver descends, the pressure on their lungs increases, so the air volume inside the lungs must decrease |
| Application to SCUBA diving | As a diver ascends, the pressure on their thoracic cage decreases, so the volume of air inside the lungs increases |
What You'll Learn

Boyle's Law and inhalation
Boyle's Law, discovered by Robert Boyle in 1662, is a gas law that describes the relationship between the pressure and volume of a gas. The law states that the pressure exerted by a gas is inversely proportional to its volume when the temperature and amount of gas remain constant. This is represented by the equation PV = K, where P is pressure, V is volume, and K is a constant.
Boyle's Law is fundamental to understanding the human respiratory system and the process of inhalation. When we inhale, our diaphragm contracts, and the external intercostal muscles elevate the ribs and sternum, causing the lungs to expand. This increase in lung volume leads to a decrease in air pressure inside the lungs, as described by Boyle's Law. As a result, air flows from the higher pressure outside the body into the lower pressure inside the lungs, facilitating inhalation.
During inhalation, the inspiratory muscles, including the diaphragm and external intercostal muscles, play a crucial role. Their contraction increases the volume of the thoracic cavity and the lungs, leading to a decrease in intrapleural pressure. This pressure reduction allows air to flow into the lungs for gas exchange. At rest, the alveolar pressure is equal to atmospheric pressure. However, during inhalation, as the volume expands within the alveoli, the alveolar pressure drops below atmospheric pressure, facilitating air inflow.
It is important to note that the lungs do not always follow Boyle's Law at all volumes. At low lung volumes, a significant pressure change is required to make small volume changes, while at high volumes, it takes more negative pressure to expand the tissue. Nonetheless, during normal breathing with a tidal volume, the lungs follow the proportional changes in volume and pressure as described by Boyle's Law.
Understanding Boyle's Law is not limited to breathing but also has practical applications in various fields. For example, it explains the operation of medical syringes and is crucial for SCUBA divers to comprehend as they experience changes in pressure and volume when descending and ascending underwater.
Portland Rental Law: Urban Growth Boundary Rules Explained
You may want to see also

Boyle's Law and exhalation
Boyle's law, a gas law formulated by Robert Boyle in 1662, explains the relationship between the pressure and volume of a gas. The law states that, when the temperature and amount of gas remain constant, the pressure and volume of a gas are inversely proportional. This means that as the volume of a gas increases, its pressure decreases, and vice versa.
The human respiratory system functions according to Boyle's law. During inhalation, the lungs expand, leading to an increase in volume and a decrease in pressure inside the lungs. As a result, air from the outside, which is at a higher pressure, moves into the lungs.
During exhalation, the opposite process occurs. The volume inside the lungs decreases as they deflate, leading to an increase in pressure. This increase in pressure forces the air out of the lungs and back into the atmosphere.
The process of exhalation can be understood by considering the relationship between pressure and volume described by Boyle's law. As the volume of gas in the lungs decreases during exhalation, the pressure increases. This increase in pressure creates a pressure gradient between the inside of the lungs and the atmosphere, with the pressure inside the lungs becoming higher than the atmospheric pressure. According to the principles of gas exchange, gas flows from an area of high pressure to an area of low pressure. Therefore, the air inside the lungs, now at a higher pressure, moves out of the lungs and into the atmosphere, which is at a lower pressure.
It is important to note that the lungs do not always follow Boyle's law exactly. At low and high lung volumes, the compliance of the lung tissue, or its ability to expand, decreases. This means that it takes a larger pressure change to make small volume changes at low and high lung volumes. However, during a resting state with a normal tidal volume, the lungs generally follow the proportional changes in volume and pressure as described by Boyle's law.
Understanding Colorado Chain Laws: Do They Apply to Cars?
You may want to see also

Lung compliance
The lungs do not follow Boyle's law at all volumes. Boyle's law, which states that pressure and volume are inversely proportional, is only applicable when the lungs are in a resting state with a normal tidal volume, and the alveoli are not collapsed nor are the lungs at maximal capacity. At these volumes, the lungs follow proportional changes of volume and pressure per Boyle's law.
At birth, lung compliance is low as newborns are born with no air within their alveoli. As a result, the effort to create negative intrapleural pressure during the initial breaths is high. However, as the lungs fill with air and become more compliant with successive breaths, they begin to follow Boyle's law, exhibiting an inverse relationship between pressure and volume.
California Muscle Cars: Exempt from Exhaust Laws?
You may want to see also

The respiratory system
The human body brings air into the lungs by negative pressure. In a resting state, the thoracic cavity is in static equilibrium with an intrapleural pressure of approximately -5 cm H2O. During inhalation, the inspiratory muscles, namely the diaphragm and external intercostal muscles, contract and relax, respectively, increasing the volume of the thoracic cavity. This contraction causes the lungs to expand, and as per Boyle's law, the increase in volume leads to a decrease in pressure within the lungs. As a result, air flows from the higher pressure outside the body into the lungs, facilitating gas exchange.
During exhalation, the inspiratory muscles relax, decreasing the volume within the thorax and increasing the pressure. This pressure forces the alveolar air back out into the atmosphere. Thus, during the breathing cycle, the volume and pressure inside the lungs change, following Boyle's law.
Boyle's law also has clinical significance in respiratory conditions such as pneumothorax, where increased pressure within the intrapleural space affects the ability to create negative pressure and draw air into the lungs. Additionally, it is essential for understanding the mechanics of breathing in activities like scuba diving, where changes in pressure at different depths can impact the volume of air in the lungs.
FMLA Laws: Do Foreign Companies Need to Comply?
You may want to see also

Gas laws
Boyle's Law applies when the temperature does not change. It states that if the volume of a gas increases, the pressure must decrease, and vice versa. This is often referred to as an inverse relationship.
The human respiratory system functions according to Boyle's Law. When we breathe in, our diaphragm lowers, increasing the volume inside our lungs. This, in turn, decreases the air pressure inside our lungs, so that outside air is drawn into our lungs. Conversely, when we breathe out, our diaphragm pushes upwards, reducing the volume inside our lungs, which increases the pressure and forces air outwards.
Boyle's Law also applies in other situations. For example, it explains how a syringe works. When the plunger of a syringe is pulled out, the volume inside the barrel increases, resulting in a decrease in pressure. Fluids outside the syringe are then drawn in to balance the pressure. Similarly, scuba divers must be aware of Boyle's Law as they change depth underwater. As a diver descends, the pressure on their lungs increases, so the air volume inside their lungs must decrease according to Boyle's Law. As the diver ascends, the pressure decreases, and the volume of air inside their lungs increases.
Stark Law and Its Applicability to Medicaid Patients
You may want to see also
Frequently asked questions
Boyle's Law is a gas law that describes the relationship between the pressure and volume of a gas for a fixed mass and temperature. It is represented by the equation PV = K, where P is pressure, V is volume, and K is a constant.
When we inhale, our diaphragm lowers, increasing the volume inside our lungs. According to Boyle's Law, as the volume increases, the pressure inside the lungs decreases. This causes outside air to be drawn into the lungs as air moves from high to low pressure.
During exhalation, or breathing out, the diaphragm pushes upwards, reducing the volume inside the lungs and increasing the pressure. This forces the air out of the lungs.
No, the lungs do not follow Boyle's Law at all volumes. At low and high volumes, the lung has low compliance, meaning the tissue's ability to expand or its elasticity decreases.
Boyle's Law can explain the working of a syringe and how SCUBA divers experience changes in pressure and volume as they descend and ascend underwater.







