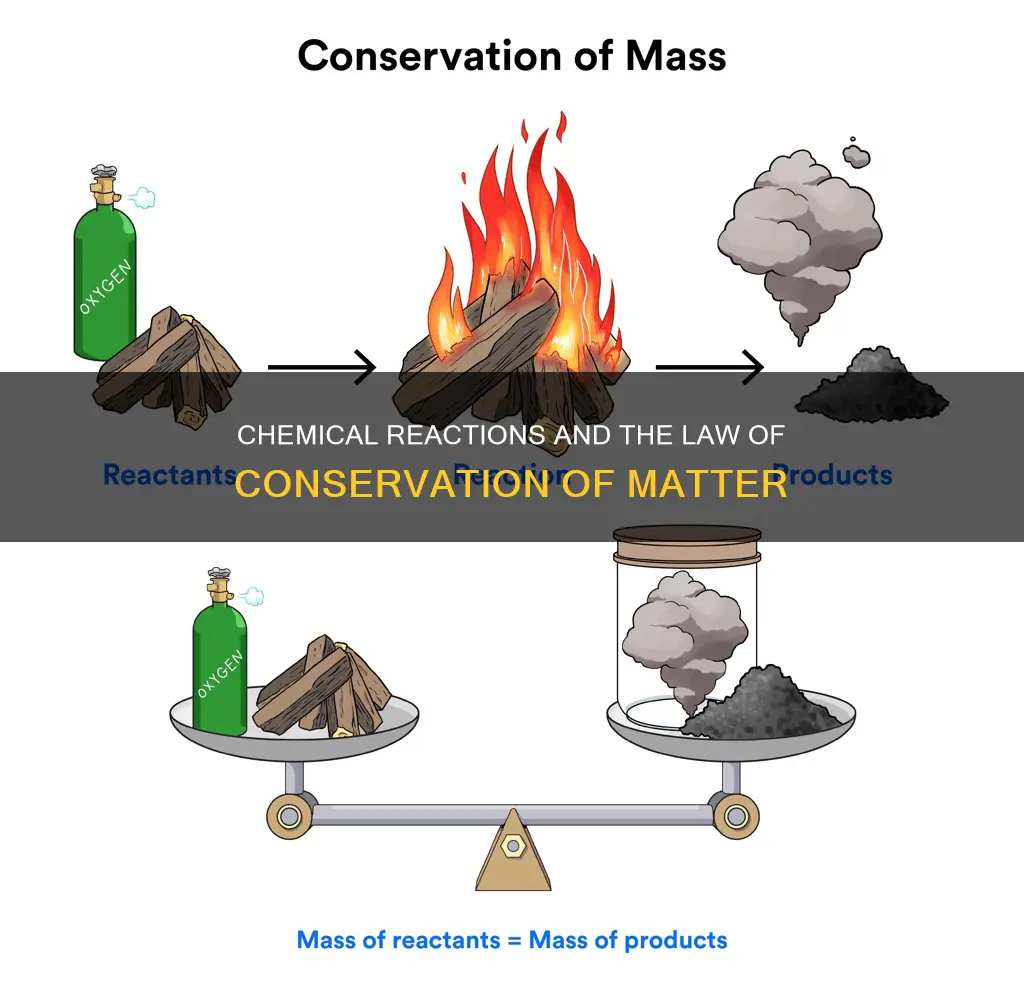
The law of conservation of matter, also known as the law of indestructibility of matter, states that in a closed system, the amount of matter remains constant. In other words, matter is neither created nor destroyed. This law applies to chemical reactions, where the mass of the products must equal the mass of the reactants. For example, when wood burns, it combines with oxygen to form ashes, carbon dioxide, and water vapour. While it may seem like burning destroys matter, the total mass of the products after the fire is equal to the total mass before it. This principle forms the basis of modern chemistry and is used in many fields, including mechanics and fluid dynamics.
| Characteristics | Values |
|---|---|
| Mass of reactants | = |
| Mass of products | Mass of reactants |
| Mass in a closed system | Conserved |
| Mass in open systems | Not conserved |
| Mass in high-energy systems | Not conserved |
What You'll Learn
- The law of conservation of matter states that the amount of matter in a closed system remains constant
- Mass is neither created nor destroyed during chemical reactions
- The total mass of the reactants equals the total mass of the products
- Mass is conserved in every chemical reaction, though losses occur during handling
- The law of conservation of matter is also known as the law of indestructibility of matter

The law of conservation of matter states that the amount of matter in a closed system remains constant
The law of conservation of matter, also known as the law of indestructibility of matter, states that in a closed system, the amount of matter remains constant. This means that matter cannot be created or destroyed, only rearranged. In the context of chemical reactions, this law implies that the mass of the chemical components before the reaction must be equal to the mass of the components after the reaction.
For example, in the chemical equation: CH4 + 2O2 → CO2 + 2H2O, one molecule of methane (CH4) and two molecules of oxygen (O2) are converted into one molecule of carbon dioxide (CO2) and two molecules of water (H2O). The number of molecules produced must be equal to the number of molecules initially present, as the total mass of the reactants is conserved and remains constant throughout the reaction.
The law of conservation of matter was discovered by Antoine Lavoisier in 1789 and laid the foundation for modern chemistry. It is based on the observation that naturally occurring elements are very stable under typical conditions found on Earth. Most elements originate from fusion reactions in stars or supernovae, and on Earth, they do not convert into other elements during chemical reactions. This stability ensures that the law of conservation of matter holds true for chemical reactions, as the individual atoms that make up matter remain constant and only change form or rearrange.
The law of conservation of matter has significant implications for various fields, including chemistry, mechanics, and fluid dynamics. It is essential for understanding chemical reactions and stoichiometry, as it provides the basis for balancing chemical equations. Additionally, the law of conservation of matter has been applied to the analysis of elemental cycles in ecology through mass balance studies. These studies help ecologists understand the movement of elements through ecosystems and the impact of human activities on these cycles.
Trademark Law: Historic Company Depictions and Legal Boundaries
You may want to see also

Mass is neither created nor destroyed during chemical reactions
The law of conservation of mass, also known as the law of indestructibility of matter, states that mass is neither created nor destroyed during any physical or chemical change. In other words, the total mass of the products of a chemical reaction remains equal to the total mass of the reactants. This principle was discovered by Antoine Lavoisier in 1789 and laid the foundation for modern chemistry.
According to the law of conservation of mass, the mass of an element at the beginning of a reaction will be the same as the mass of that element at the end of the reaction. This means that the total mass of all the reactants and products in a closed system remains the same at any point in time. For example, when wood burns, it combines with oxygen to form ashes, carbon dioxide, and water vapour. While it may seem like burning destroys matter, the total mass of the wood before burning is equal to the total mass of the resulting ashes, gases, and air.
The law of conservation of mass holds true because naturally occurring elements are very stable under the conditions found on Earth. Most elements are formed from fusion reactions that only occur in stars or supernovae. Therefore, under typical conditions on Earth, atoms are not converted into other elements during chemical reactions. Instead, individual atoms cycle through various chemical compounds. For example, a carbon atom may be buried as coal for millions of years, then burned in a power plant, released into the atmosphere, dissolved in the ocean, and eventually taken up by an algal cell.
The concept of mass conservation is widely applied in fields such as chemistry, mechanics, and fluid dynamics. It is also used to study ecosystems, where scientists track the flow of elements through various compartments, such as a forest or a pool of carbon. By accounting for all inputs and outputs, scientists can analyse the mass balance of elements in different ecosystems.
Lemon Law: Private Sellers and You
You may want to see also

The total mass of the reactants equals the total mass of the products
The law of conservation of mass, also known as the law of indestructibility of matter, states that in any closed system, the amount of matter in the system remains constant. In other words, the total mass of the reactants equals the total mass of the products.
This law applies to any system that is closed to the transfer of matter and energy. In such a system, the mass of the system must remain constant over time. This implies that mass can neither be created nor destroyed, only rearranged or changed in form. For example, in a chemical reaction, the mass of the reactants before the reaction must be equal to the mass of the products after the reaction.
The law of conservation of mass was discovered by Antoine Lavoisier in 1789 and laid the foundation for modern chemistry. It holds true because naturally occurring elements are very stable at the conditions found on Earth. Most elements come from fusion reactions found only in stars or supernovae, so on Earth, atoms are not typically converted to other elements during chemical reactions.
The law of conservation of mass can be applied to various fields, including chemistry, mechanics, and fluid dynamics. It is also essential in understanding chemical equations and stoichiometry, which is the calculation of the amount of reactant and products in a chemical reaction.
In summary, the law of conservation of mass states that the total mass of the reactants equals the total mass of the products in any closed system. This law has revolutionized science and is fundamental in various scientific disciplines.
Stark Law and LMHCs: Understanding the Legal Boundaries
You may want to see also

Mass is conserved in every chemical reaction, though losses occur during handling
The law of conservation of matter, also known as the law of indestructibility of matter, states that in a closed system, the amount of matter in the system remains constant over time. In other words, matter is neither created nor destroyed, only rearranged. This law applies to both physical and chemical changes, and it holds true for all chemical reactions.
According to the law of conservation of mass, the total mass of the products in a chemical reaction must be equal to the total mass of the reactants. This means that the mass of an element at the beginning of a reaction will be the same as the mass of that element at the end of the reaction. For example, in the reaction:
> CH4 + 2 O2 → CO2 + 2 H2O
The mass of methane (CH4) and oxygen (O2) at the start of the reaction is the same as the mass of carbon dioxide (CO2) and water (H2O) at the end.
While mass is conserved in every chemical reaction, losses can occur during handling. This is because, for every reactant particle, there must be a corresponding product particle with an equivalent mass. However, in practice, it is rare to achieve a 100% yield, and some reactant particles may be lost or not fully converted into products.
The law of conservation of mass was discovered by Antoine Lavoisier in 1789 and laid the foundation for modern chemistry. It is based on the idea that naturally occurring elements are very stable under normal conditions on Earth. Most elements come from fusion reactions in stars or supernovae, so on Earth, atoms are not typically converted into other elements during chemical reactions. This means that individual atoms can have very long histories as they cycle through different chemical compounds.
Inertia's Law: Universal or Selective Applicability?
You may want to see also

The law of conservation of matter is also known as the law of indestructibility of matter
The law of conservation of matter, also known as the law of indestructibility of matter, is a fundamental principle in science, particularly in physics and chemistry. This law states that in a closed system, the amount of matter remains constant over time, implying that matter cannot be created or destroyed, only transformed.
The law of conservation of matter can be applied to chemical reactions. In any chemical reaction, the total mass of the reactants (starting materials) is equal to the total mass of the products. This principle is known as the law of conservation of mass. For example, in the chemical reaction where methane and oxygen are converted into carbon dioxide and water:
> CH4 + 2 O2 → CO2 + 2 H2O
The mass of the chemical components before the reaction is equal to the mass of the components after the reaction. Thus, the law of conservation of mass demonstrates the application of the law of conservation of matter in chemical reactions.
The concept of the indestructibility of matter has a long history in philosophy and science. As early as 520 BCE, Jain philosophy, based on the teachings of Mahavira, asserted that matter cannot be created or destroyed. Similarly, ancient Greek philosophy espoused the idea that "nothing comes from nothing," implying that existing matter has always existed and cannot be utterly destroyed.
The law of conservation of matter, or the law of indestructibility of matter, has significant implications for our understanding of the natural world. It provides a foundation for scientific investigation, particularly in chemistry, and has played a crucial role in the development of modern science.
Humanitarian Law: Civil War and International Application
You may want to see also
Frequently asked questions
The law of conservation of matter, also known as the law of indestructibility of matter, states that in a closed system, the amount of matter in the system remains constant over time.
The law of conservation of matter applies to chemical reactions by stating that the mass of the reactants will be equal to the mass of the products. In other words, matter is neither created nor destroyed during a chemical reaction, only transformed.
Yes, consider the following chemical reaction:
CH4 + 2O2 → CO2 + 2H2O
In this reaction, one molecule of methane (CH4) and two molecules of oxygen (O2) combine to form one molecule of carbon dioxide (CO2) and two molecules of water (H2O). The law of conservation of matter dictates that the total mass of the reactants (methane and oxygen) will be equal to the total mass of the products (carbon dioxide and water).