Gas laws are a crucial aspect of understanding the principles behind submarines and diving. In particular, Boyle's Law, which states that the volume of a gas varies inversely with pressure at a constant temperature, while gas density varies directly with pressure, has important implications for submarine operations and diver safety. As a submarine descends, the pressure exerted on it increases, causing changes in gas volume and density within the vessel. Similarly, a diver's body is subjected to these pressure changes, which can lead to dangerous consequences if not properly managed. Understanding gas laws is, therefore, essential to ensure the safety and efficient operation of both submarines and divers underwater.
What You'll Learn

Boyle's Law and gas density
Gas laws, such as Boyle's Law, are crucial in understanding the principles behind the functioning of submarines. Submarines operate by manipulating the delicate balance between pressure and volume, ensuring neutral buoyancy to descend or ascend smoothly.
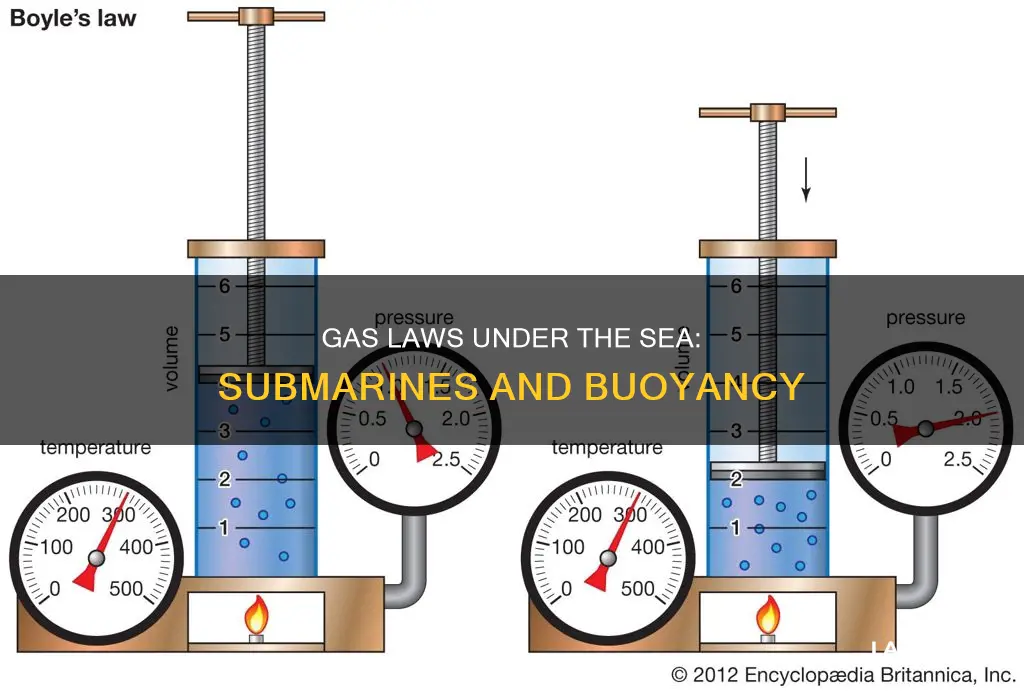
Boyle's law, established by Anglo-Irish chemist Robert Boyle in 1662, is a fundamental principle in gas behaviour. It states that the pressure exerted by a gas is inversely proportional to the volume it occupies, provided the temperature and the quantity of gas remain constant. This relationship can be expressed mathematically as:
P ∝ (1/V)
Or
P1V1 = P2V2
Where P is pressure, V is volume, and the subscripts represent initial and final states. This law indicates that as volume increases, pressure decreases, and vice versa.
The law is based on experiments conducted by Boyle, where he used a closed J-shaped tube and mercury to study the behaviour of air. He discovered that under controlled conditions, the pressure of a gas is inversely related to its volume.
Boyle's law has significant implications for submarine operations. As a submarine submerges, the pressure around it increases. To maintain neutral buoyancy and avoid being crushed by the surrounding water pressure, the submarine must adjust its volume. This is achieved by flooding specific compartments with seawater, effectively reducing the volume of the submarine and increasing its pressure to match that of the surrounding water.
Similarly, when a submarine resurfaces, it must adjust its volume again. This time, it pumps out the seawater, increasing its volume and reducing the pressure to match the lower pressure of the surface.
Boyle's law also has implications for the air inside a submarine. As the submarine submerges and the pressure around it increases, the air inside the submarine is compressed, leading to a decrease in volume. This compression can be dangerous, and submarines are designed with strong hulls to withstand these pressure changes.
Additionally, Boyle's law can explain the operation of buoyancy control tanks in submarines. These tanks are used to adjust the overall buoyancy of the vessel by taking in or expelling water. By manipulating the volume and pressure of the water in these tanks, submarines can achieve neutral buoyancy and precise depth control.
In summary, Boyle's law, with its relationship between pressure and volume, is essential for understanding the principles behind submarine operations. Submarines rely on carefully manipulating volume and pressure to maintain structural integrity, control buoyancy, and enable smooth descent and ascent.
Libel Law Complexities: Public Figures and Legal Boundaries
You may want to see also

Gas volume and pressure
Boyle's Law states that at a constant temperature, the volume of a gas is inversely proportional to its pressure. In the context of submarines, this means that as a submarine dives deeper into the ocean, the pressure exerted on the submarine increases, causing the volume of the gas inside to decrease. This principle is crucial for maintaining the structural integrity of the submarine and ensuring the safety of its occupants.
Charles's Law, on the other hand, relates the volume of a gas to its temperature at a constant pressure. According to this law, the volume of a gas increases as its temperature increases, and vice versa. This has important implications for submarines, especially when it comes to gas storage and propulsion systems. For example, the dive shop owners fill the gas tanks with water at a lower temperature to ensure the gas pressure is lower than expected.
The application of these gas laws becomes even more critical when considering the air spaces within a diver's body. As a diver descends into the water, the pressure exerted on their body increases, causing the volume of air in their lungs and other air-filled cavities to decrease according to Boyle's Law. This can lead to serious health risks, such as sinus or ear pain, and even lung rupture if a diver ascends too quickly without properly equalizing the pressure.
Additionally, the increased pressure at greater depths can affect the density of the air a diver breathes. According to gas laws, as pressure increases, the density of the air also increases, leading to higher gas absorption. This can result in decompression sickness if a diver ascends too quickly or does not follow proper decompression procedures. Therefore, a thorough understanding of gas volume and pressure relationships is essential for safe diving practices and submarine operation.
Grandfather Law: Are Old Septic Systems Still Legal?
You may want to see also

Gas mixtures and pressure
Let's begin with Boyle's Law, which states that at a constant temperature, the volume of a gas varies inversely with the pressure, while the density of a gas varies directly with pressure. In the context of submarines and diving, this law has significant implications. As a submarine descends, the pressure exerted by the water column increases, leading to a corresponding decrease in the volume of any gas-filled compartments or spaces within the submarine. This principle also applies to the air spaces in a diver's body, such as the lungs and sinuses, where pressure and volume changes occur in direct proportion to the depth.
Charles's Law is also relevant and states that at a constant volume, the pressure of a gas varies directly with its absolute temperature. For example, consider a scuba tank filled with air at a certain temperature. When submerged in water at a lower temperature, Charles's Law predicts that the tank pressure will decrease. Dive shop owners often fill tanks with gas kept at a lower temperature to account for this phenomenon.
Dalton's Law addresses gas mixtures and states that the total pressure exerted by a mixture of gases is equal to the sum of the pressures that would be exerted by each gas if it alone occupied the total volume. This law is crucial when considering the composition of air, which primarily consists of nitrogen and oxygen. At different depths, the partial pressures of these gases change, leading to potential issues like nitrogen narcosis, which can cause confusion and pose risks to divers.
Lastly, Henry's Law explains the relationship between the pressure and amount of a gas dissolved in a liquid. As a diver descends, the ambient pressure increases, leading to an increase in the partial pressures of oxygen and nitrogen in the body. Consequently, more molecules of these gases dissolve in the blood and tissues. During ascent, as the ambient pressure decreases, these dissolved gases are released from the body, primarily through the lungs. A controlled ascent rate and proper decompression stops are crucial to ensuring safe elimination of these gases.
In summary, gas laws, including Boyle's Law, Charles's Law, Dalton's Law, and Henry's Law, have direct applications in submarine and diving scenarios. Understanding these laws is essential for the safe operation of submarines and for protecting the health and well-being of divers.
Kepler's Laws: Beyond the Six Planets
You may want to see also

Gas absorption and depth
Submarines are complex machines that can dive to great depths and remain underwater for extended periods. One of the critical challenges in submarine design is managing the balance of gases within this enclosed environment. The air composition must be carefully regulated to maintain breathable conditions for the crew. This includes ensuring adequate oxygen levels while removing carbon dioxide, a by-product of human respiration.
Oxygen generation and maintenance are crucial for the survival of the crew. Submarines employ various methods to produce oxygen, including electrolysis of water, chemical oxygen concentrators, and solid polymer oxygen generators. These systems ensure a continuous supply of oxygen, which is essential for the crew's sustenance and the submarine's operation.
Carbon dioxide (CO2) removal, or "scrubbing," is equally important. CO2 accumulation beyond 5% can be harmful and even fatal. Several methods are used to achieve this, including soda lime absorption, alcohol amines (such as monomethyl amine), and lithium hydroxide absorbers. These processes trap and convert CO2, preventing its buildup and maintaining safe air quality.
The depth of a submarine impacts gas absorption and management. As a submarine descends, the pressure increases, affecting gas solubility and the rate of gas exchange. This has implications for both oxygen and CO2 management. Additionally, the confined space and lack of natural ventilation in submarines further emphasize the importance of effective gas absorption and depth-related considerations.
The complex interplay of gas laws, depth, and submarine design showcases the challenges of creating and maintaining a habitable underwater environment. Managing gas absorption and depth is a critical aspect of submarine technology, ensuring the safety and functionality of these remarkable vessels and their crews.
Clergy Confidentiality: Understanding HIPAA Law Applications
You may want to see also

Gas solubility and temperature
Gas solubility refers to the amount of gas that can dissolve in a given quantity of solvent at a specific temperature or pressure. In the context of submarines, understanding the solubility of gases, particularly oxygen, is crucial for maintaining the habitability of the submarine and ensuring the safety of its crew.
The solubility of a gas is inversely proportional to its temperature. This means that as the temperature increases, the solubility of the gas decreases. This relationship is described by Henry's Law, which states that the concentration of a dissolved gas is directly proportional to the partial pressure of that gas above the solution. In the case of a submarine, the increase in temperature can lead to a decrease in the solubility of oxygen in the water surrounding the vessel, affecting its availability for the crew.
The impact of temperature on gas solubility can be explained by Le Chatelier's Principle. This principle states that when a system is subjected to a change in temperature, the system will adjust to minimise the effect of that change. In the case of a gas dissolving in a solution, if the temperature increases, the system will respond by reducing the amount of gas that can be dissolved, resulting in a decrease in solubility.
The relationship between temperature and gas solubility has important implications for submarine operations. As the temperature inside a disabled submarine rises, the solubility of oxygen decreases, leading to a reduction in the available oxygen for the crew. This, in turn, can impact the crew's ability to survive and increase the urgency of escape or rescue.
Additionally, the temperature inside a disabled submarine can affect the crew's ability to tolerate thermal stress. High temperatures and humidity, coupled with limited air movement, can create a state of uncompensable thermal stress. This can impair the crew's cognitive function, physical performance, and decision-making abilities, further complicating the survival situation.
Employment Laws: Who Is Covered and Who Isn't?
You may want to see also
Frequently asked questions
Gas laws are crucial in understanding the behaviour of gases under different conditions of pressure and temperature. For submariners, this knowledge can be a matter of life and death. For example, Boyle's Law explains how the volume of a gas varies with pressure, which is relevant when dealing with air spaces in the body when diving or ascending from a dive.
According to gas laws, increased pressure leads to an increase in the density of air and a decrease in volume. This affects air spaces in the body, such as the sinuses, ears, and lungs. As a submarine dives deeper, the pressure increases, causing compression of gases in these air spaces, which can lead to pain and even injury if not managed properly.
Understanding gas laws is the first step to ensuring safety. Submarines should be equipped with the necessary tools to monitor and control pressure and temperature. Proper training in diving and ascending procedures, including controlled ascent rates and decompression stops, is essential to avoid issues such as decompression sickness and nitrogen narcosis, which can be dangerous for the crew.