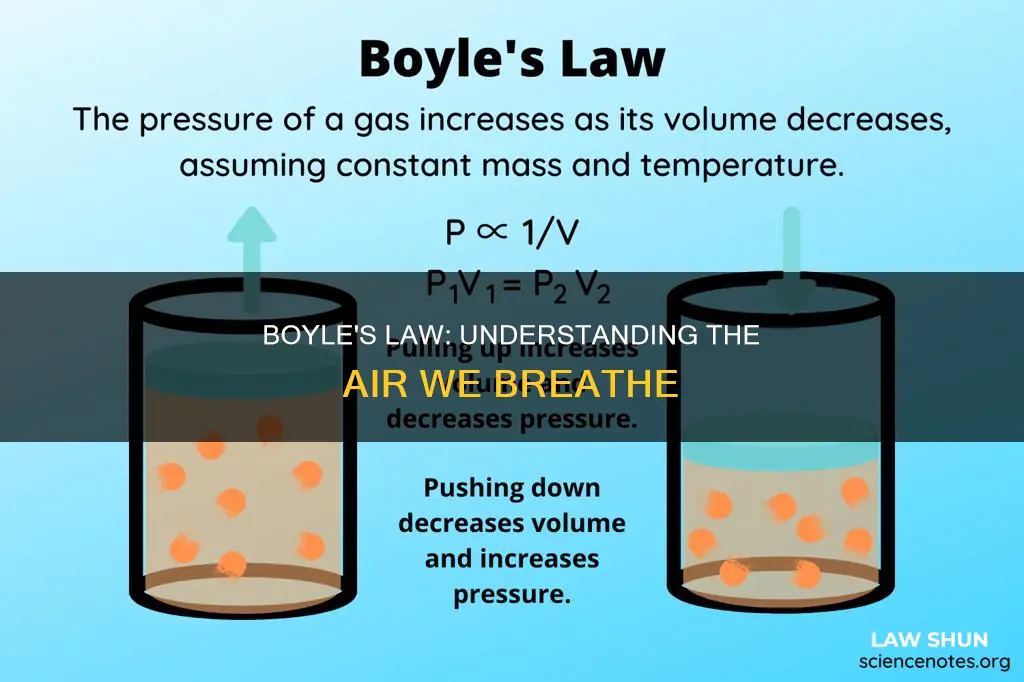
Boyle's Law, also known as the Boyle-Mariotte Law, is a fundamental principle in the field of gas behaviour, describing the inverse relationship between the pressure and volume of a confined gas. This law, formulated by Robert Boyle in 1662, states that when the temperature of a given mass of gas is held constant, any change in pressure results in a proportional variation in volume, and vice versa. The law is expressed mathematically as P1V1 = P2V2, where P and V represent initial and final pressure and volume values. This law has significant applications in various fields, including clinical situations and understanding the human breathing system.
| Characteristics | Values |
|---|---|
| Relationship | An inverse relationship between pressure and volume |
| Applicability | Only if the number of molecules (n) and the temperature (T) are both constant |
| Prediction | Used to predict the result of introducing a change in volume and pressure only, and only to the initial state of a fixed quantity of gas |
| Formula | P1V1 = P2V2, where P1 and V1 are the initial pressure and volume values, and P2 and V2 are the values of the pressure and volume of the gas after change |
| Named After | Chemist and physicist Robert Boyle |
What You'll Learn
- How does Boyle's Law apply to gases in closed cavities in the body?
- How does it explain the use of saline in the cuff of an endotracheal tube during hyperbaric therapy?
- How does it apply to the breathing system in the human body?
- How does it explain the effects of altitude on gases in closed cavities in the body?
- How can it be used to calculate the total intra-thoracic gas volume?

How does Boyle's Law apply to gases in closed cavities in the body?
Boyle's Law, or the Boyle-Mariotte Law, states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the volume occupied by it. In other words, the pressure and volume of a gas are inversely proportional to each other as long as the temperature and the quantity of gas remain constant.
Boyle's Law can be applied to gases in closed cavities in the body, such as the lungs, to understand the mechanics of breathing. The lungs do not follow Boyle's Law at all volumes, but in a resting state with a normal tidal volume, the lungs follow proportional changes in volume and pressure as dictated by the law.
As the volume of the lungs increases, the pressure must decrease, and vice versa. This inverse relationship between pressure and volume creates a pump-vacuum activity that allows us to breathe. When the diaphragm contracts and pulls downward, it expands the volume of the thoracic cavity, decreasing the pressure in the lungs and creating a vacuum. This reduction in pressure pulls air into the lungs. Conversely, when the diaphragm relaxes and is compressed upward, the volume of the thoracic cavity decreases, increasing the pressure and forcing air out of the lungs.
Boyle's Law can also be applied to understand the effects of altitude on gases in closed cavities within the body. For example, as a SCUBA diver descends into the water, the pressure on their lungs increases, and according to Boyle's Law, the air volume inside the lungs must decrease. As the diver ascends and the pressure decreases, the volume of air inside the lungs increases. If the diver does not exhale steadily to release this increased volume of air, they may experience pulmonary barotrauma, or overexpansion and alveolar rupture.
Additionally, Boyle's Law can be used to calculate the total intra-thoracic gas volume by body plethysmography. As altitude increases, ambient pressure decreases, and according to Boyle's Law, volume expansion occurs in enclosed spaces. This can be observed in the expansion of a sealed bag of potato chips on a commercial flight. Similarly, gases in the lungs can expand at higher altitudes, which can cause barotrauma, arterial gas embolism, mediastinal emphysema, or pneumothorax.
The Law and Kuwaiti Citizens: Who Does It Affect?
You may want to see also

How does it explain the use of saline in the cuff of an endotracheal tube during hyperbaric therapy?
Boyle's Law, also known as the Boyle-Mariotte Law, is an empirical gas law that describes the relationship between the pressure and volume of a confined gas. It states that the pressure exerted by a gas is inversely proportional to the volume it occupies, provided the temperature and amount of gas remain constant. This law is expressed by the equation: PV = k, where P is pressure, V is volume, and k is a constant.
Now, let's discuss how this applies to the use of saline in the cuff of an endotracheal tube during hyperbaric therapy. During hyperbaric oxygen treatment (HBOT), patients are subjected to increased ambient pressure. If the cuff of an endotracheal tube remains inflated with air, which is compressible, the cuff volume will decrease due to Boyle's Law, leading to leakage during positive pressure ventilation. On the other hand, water is non-compressible, and thus, replacing the air in the cuff with saline or sterile water before HBOT is the standard practice. This prevents leakage and protects the patient's airway.
Additionally, during decompression, an increase in cuff volume due to the compressibility of air could cause cuff rupture. Using saline or water in the cuff ensures that the volume remains constant, reducing the risk of rupture.
It is worth noting that the pressure in the cuff should be monitored and controlled. Overinflation of the cuff can lead to tracheal mucosa ischaemia and tracheal stenosis, while underinflation can result in micro-inhalations and ventilator-associated pneumonia. Therefore, maintaining the recommended cuff pressure is crucial for patient safety.
HIPAA Compliance During COVID-19: What You Need to Know
You may want to see also

How does it apply to the breathing system in the human body?
Boyle's law states that the volume of a gas and pressure are inversely proportional at a given temperature. In other words, when the pressure increases, the volume decreases, and vice versa. This law is important because it helps us understand the relationship between pressure and volume in the lungs when breathing.
The lungs do not always follow Boyle's law. At rest, with a normal tidal volume, the lungs follow proportional changes in volume and pressure as per Boyle's law. However, at low and high volumes, the lung has low compliance, meaning the tissue's ability to expand or its elasticity decreases.
During inhalation, the diaphragm contracts and pulls downward toward the abdominal cavity, expanding the volume of the thoracic cavity. This decreases the pressure in the lungs and creates an empty space, forming a vacuum. This reduction in pressure pulls air into the lungs. This air can enter the respiratory tract from the nasal cavities or mouth, travelling to the pharynx, larynx, trachea, bronchi, and bronchioles, before reaching the alveoli to diffuse oxygen and carbon dioxide.
When the inspiratory muscles relax, the volume within the thorax decreases, and the pressure increases, forcing alveolar air back out into the atmosphere. As a result of this process, inhalation and exhalation occur, allowing humans to breathe.
The inverse relationship between pressure and volume described by Boyle's law creates the pump-vacuum activity that enables breathing. This law is particularly relevant in the pleural cavity, which is enclosed and experiences pressure and volume changes as the lungs expand and contract.
Understanding Blue Laws: Who Does It Affect?
You may want to see also

How does it explain the effects of altitude on gases in closed cavities in the body?
Boyle's Law states that the absolute pressure and volume of a given mass of confined gas are inversely proportional, provided the temperature remains unchanged within a closed system. This law can be used to explain the effects of altitude on gases in closed cavities in the body.
As altitude increases, the pressure exerted on a gas inside a closed space decreases, leading to volume expansion. This is because, according to Boyle's Law, when the pressure on a gas decreases, its volume increases, and vice versa. This expansion can be significant, reaching up to 20% at an altitude of 8,000 feet.
One example of this effect is the expansion of a sealed bag of potato chips during a commercial flight. Another example is the increase in volume of a pneumothorax during airlifting a patient to a hospital. A pneumothorax is a clinical condition where air or gas accumulates in the space between the lungs and the chest wall, creating pressure that can cause lung collapse. As the aircraft gains altitude, the pressure inside the aircraft decreases, leading to an expansion of the pneumothorax volume. This can have serious consequences for the patient and may require medical intervention.
Boyle's Law also explains the mechanism of breathing. As the chest expands due to the movement of the thoracic cage and diaphragm, the volume of the chest cavity increases, leading to a decrease in pressure. This decrease in pressure causes air to rush into the lungs until the pressure equalizes with the atmospheric pressure.
In summary, Boyle's Law explains the effects of altitude on gases in closed cavities in the body by describing the inverse relationship between pressure and volume. As altitude increases and pressure decreases, the volume of gases in closed cavities, such as the lungs or a pneumothorax, will expand. This expansion can have important implications for both healthy individuals and patients with respiratory conditions.
Law Firms and HIPAA: What's the Deal?
You may want to see also

How can it be used to calculate the total intra-thoracic gas volume?
Boyle's law, also known as the Boyle-Mariotte law, is an empirical gas law that describes the relationship between the pressure and volume of a confined gas. It was formulated by Anglo-Irish chemist Robert Boyle in 1662 and can be stated as:
> The absolute pressure exerted by a given mass of an ideal gas is inversely proportional to the volume it occupies if the temperature and amount of gas remain unchanged within a closed system.
Mathematically, this can be expressed as:
P ∝ 1/V
Or
PV = K, where K is a constant.
Boyle's law can be used to calculate the total intra-thoracic gas volume by body plethysmography. This involves measuring the volume of air in the thoracic cavity, which includes the lungs and surrounding areas. Here's how it works:
During inspiration, the inspiratory muscles, including the diaphragm and external intercostal muscles, contract, increasing the volume of the thoracic cavity. According to Boyle's law, as the volume of the thoracic cavity increases, the pressure inside it decreases. This decrease in pressure allows air to flow into the lungs for gas exchange.
During expiration, the inspiratory muscles relax, and the volume of the thoracic cavity decreases, leading to an increase in pressure. This increase in pressure forces the air out of the lungs back into the atmosphere.
By measuring the changes in volume and pressure during inspiration and expiration, it is possible to calculate the total volume of gas in the thoracic cavity using Boyle's law. This calculation can be especially important in clinical situations, such as for patients with respiratory conditions or those undergoing hyperbaric therapy.
Cell Phone Laws: Parking Lot Exempt?
You may want to see also







