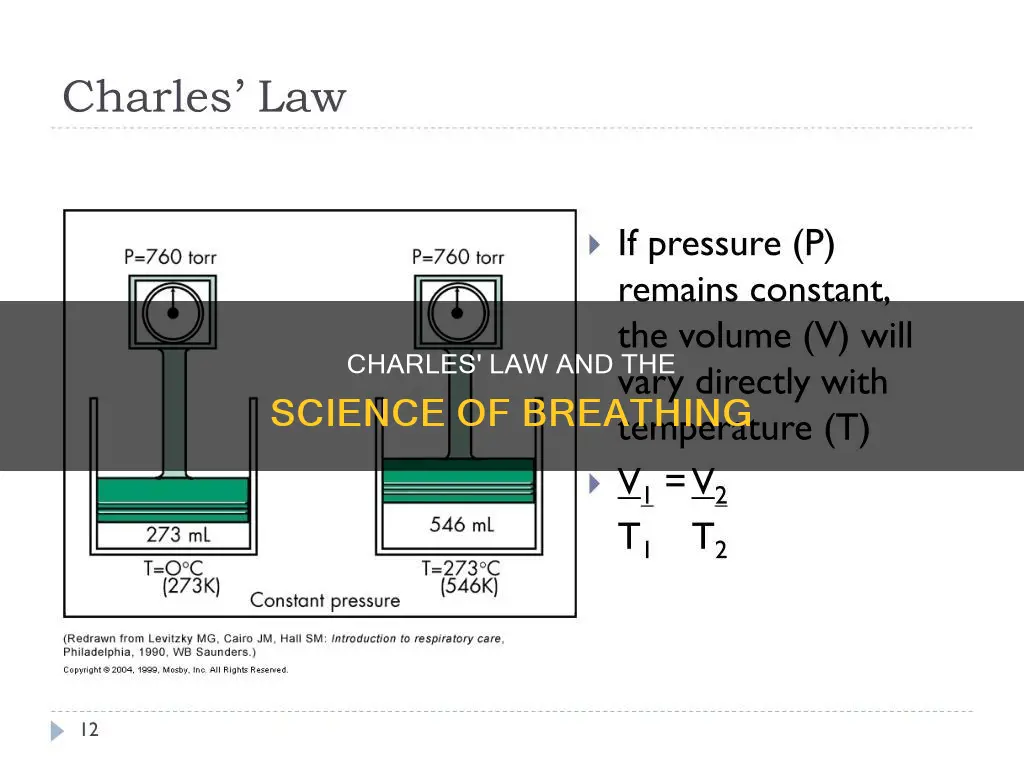
Charles's Law, also known as the law of volumes, states that when the pressure on a sample of dry gas is held constant, the Kelvin temperature and the volume are in direct proportion. In other words, as the temperature of a gas increases, so does its volume, and vice versa. This law was formulated by Jacques Charles in the 1780s and later confirmed by Joseph Louis Gay-Lussac in 1802. Although Charles's Law is the least applicable of the gas laws in terms of respiration, as body temperature rarely fluctuates, it still has an effect on breathing. When we inhale cold air, it expands as it warms in our sinuses and passes through our respiratory system. This means that we take shorter breaths in cold weather to account for the increased volume of air.
| Characteristics | Values |
|---|---|
| Effect on breathing | Affects the volume of air inhaled |
| Does not affect breathing as much as Boyle's Law | |
| Relation to temperature | As temperature increases, volume of gas also increases |
| As temperature decreases, volume of gas decreases | |
| Relation to pressure | As pressure increases, volume of gas decreases |
| As volume of container increases, pressure of gas within container decreases | |
| As volume of container decreases, pressure of gas within container increases |
What You'll Learn

Charles' Law and the volume of air inhaled
Charles's Law, also known as the law of volumes, is an experimental gas law that explains how gases tend to expand when heated. It states that when the pressure on a sample of dry gas remains constant, the Kelvin temperature and volume are directly proportional. In other words, as the temperature of a gas increases, so does its volume, and vice versa. This relationship can be expressed as:
> V ∝ T
Or:
> V = kT
Where V is the volume of the gas, T is the temperature in Kelvins, and k is a constant for a particular pressure and amount of gas.
Charles's Law can be applied to breathing to understand how the volume of air we inhale is affected by changes in temperature. When we breathe, the air we inhale passes through our sinuses and into our lungs, where it reaches a temperature of 37°C. If we inhale cold air, it will expand as it warms up to match the temperature in our lungs. For example, on a cold day with a temperature of -10°C, the volume of cold air we inhale will expand as it reaches the warmer temperature in our lungs. This means that we end up inhaling a larger volume of air than we would on a hot summer day when the outside temperature matches our body temperature.
Charles's Law demonstrates that the volume of air we inhale is dependent on the temperature of the air. As the temperature decreases, the volume of air we inhale decreases, and we take shorter breaths to compensate for the increased volume of inspired air. Conversely, as the temperature increases, the volume of air we inhale increases. Therefore, Charles's Law explains how the volume of air we inhale is influenced by the temperature of the surrounding air.
While Charles's Law provides insight into how temperature affects the volume of inhaled air, it is less applicable to respiration than other gas laws like Boyle's Law and Dalton's Law because body temperature typically remains relatively constant. However, it is important to consider the impact of temperature on breathing, especially in extreme weather conditions, to fully understand respiratory mechanics.
Sex Offender Laws: California's Community Notification Requirements
You may want to see also

How Charles' Law affects breathing
Charles's Law, also known as the law of volumes, is an experimental gas law that describes how gases tend to expand when heated. The law states that when the pressure on a sample of dry gas remains constant, the Kelvin temperature and the volume will be in direct proportion. In other words, as the temperature of a gas increases, so does its volume, and vice versa. This relationship can be expressed as:
> V ∝ T
>
> V/T = k or V = kT
Where V is the volume of the gas, T is the temperature of the gas measured in Kelvins, and k is a constant for a particular pressure and amount of gas.
Charles's Law affects breathing, although not as much as Boyle's Law. When we breathe, we inhale and exhale air, and the volume of air we inhale can be impacted by Charles's Law. For example, on a cold day, the cold air we inhale will expand as it warms up to our body temperature. This means that we end up inhaling a larger volume of air than expected. Conversely, on a hot day, the air temperature outside and inside our lungs is the same, so there is no change in volume.
Charles's Law also comes into play when considering the air pressure inside our lungs. Our lungs are a series of tubes that end in tiny sacs called alveoli, where gases are exchanged. When we inhale, the diaphragm moves down, expanding the lungs and causing a slight decrease in air pressure inside them. This decrease in pressure allows outside air to rush in, and we inhale. When we exhale, the diaphragm relaxes, and the volume of the lungs decreases, leading to an increase in pressure, forcing the air out.
While body temperature typically doesn't fluctuate much, Charles's Law demonstrates how the volume of air we inhale can be influenced by the temperature of the air we breathe in, and how this, in turn, affects our breathing.
Understanding ADA Laws During Company Sales and Acquisitions
You may want to see also

How the body temperature affects the application of Charles' Law
Charles's Law, also known as the law of volumes, is an experimental gas law that explains the relationship between the temperature and volume of a gas at constant pressure. The law states that the volume of a gas is directly proportional to its temperature in Kelvin. In other words, as the temperature of a gas increases, so does its volume, and vice versa. This law was formulated by Jacques Charles in the 1780s and has several practical applications in everyday life.
The human body inhales cold air, which then passes through the respiratory tract, warming up and causing a change in volume. This phenomenon is explained by Charles's Law. When we inhale cold air, it increases in volume as it warms up in our sinuses and reaches the temperature in our lungs. This means that on colder days, we need to take shorter breaths to account for the increased volume of air we are inhaling. Conversely, on hot summer days, the air temperature may be the same as the temperature inside our lungs, resulting in no change in volume.
Charles's Law also has implications for respiratory care. For example, it can be applied to alleviate symptoms associated with cystic fibrosis (CF) by raising the temperature of compressed oxygen and instilling water vapour into the inspired air. This technique helps to mobilise thick, viscous secretions that block airways and harbour infections. Additionally, understanding Charles's Law is crucial when working with compressed oxygen, as it confirms the relationship between the volume and temperature of the gas.
The law also has broader implications for human activity. For instance, in the summer season, our lungs can accommodate a larger volume of air compared to winter, enabling us to perform physical activities more efficiently in warm weather. Furthermore, Charles's Law can explain why bubbles spill out of a warm soda can when opened, as the volume of gas increases, causing the gas molecules to escape more readily.
Shotgun Exemption: Colorado's Unique Handgun Law Loophole
You may want to see also

The inverse relationship between volume and pressure
Charles's Law, also known as the law of volumes, describes the direct relationship between the volume and temperature of a gas. When the pressure on a sample of dry gas remains constant, the Kelvin temperature and volume will be in direct proportion. This means that as the temperature of a gas sample increases, its volume increases, and as the temperature decreases, so does the volume.
The application of Boyle's Law is evident in the act of breathing. The lungs are a series of ever-narrowing tubes that end in a myriad of tiny sacs called alveoli. The pressure change necessary for breathing is caused by the diaphragm, a muscle that covers the bottom of the lungs. When the diaphragm moves down, it expands the size of our lungs, causing a slight decrease in air pressure inside our lungs. This decrease in pressure forces new air to rush in, and we inhale. Conversely, exhaling air requires that we relax the diaphragm, which pushes against the lungs and slightly decreases the volume of the lungs. This, in turn, slightly increases the pressure of the air in the lungs, and we exhale.
Therefore, while Charles's Law does affect the volume of air we can inhale, Boyle's Law has a more significant influence on the breathing process.
Black Holes: Beyond the Laws of Physics?
You may want to see also

How Charles' Law compares to Boyle's Law
Charles's Law and Boyle's Law are two important laws that describe the behaviour of gases. They are widely used in fields such as chemistry, thermodynamics, aviation and even military applications.
Boyle's Law
Boyle's Law, proposed in 1662 by the chemist and physicist Robert Boyle, states that for a fixed amount of an ideal gas, kept at a fixed temperature, pressure and volume are inversely proportional. In other words, PV = K, where P is the pressure, V is the volume, and K is the constant. This means that if the pressure of a system is doubled, the volume of that system will be halved.
Charles's Law
Charles's Law, first published by the French philosopher Joseph Louis Gay-Lussac, states that for a closed ideal gas system under constant pressure, the volume of the system is directly proportional to the temperature of the system. In other words, V/T = K, where V is the volume of the gas and T is the temperature of the gas. It is important to note that this proportionality only works mathematically for the Kelvin scale, which is an absolute temperature scale.
Comparison
The key difference between the two laws is that Charles's Law is defined for a system with constant pressure, while Boyle's Law is defined for a system with constant temperature. The two terms in Charles's Law are directly proportional to each other, whereas the terms in Boyle's Law are inversely proportional.
Both laws involve volume, but one involves pressure and the other temperature. Charles's Law states that as the temperature of the molecules increases, they move faster, creating more pressure on the container of the gas and increasing the volume if the pressure remains constant. On the other hand, Boyle's Law states that as the volume decreases, molecules collide more frequently, creating more pressure.
Equal Protection: Criminal and Civil Law
You may want to see also
Frequently asked questions
Charles's Law states that when the pressure on a sample of dry gas is held constant, the Kelvin temperature and the volume are in direct proportion. In other words, as the temperature of a gas increases, so does its volume, and vice versa. This law applies to breathing because the air we inhale changes volume as it warms in our sinuses and lungs.
When we inhale cold air, it will expand as it warms up to our body temperature. For example, if the outside temperature is -10°C and the temperature in our lungs is 37°C, we would have to inhale 420 mL of cold air to make a volume of 500 mL at 37°C.
Charles's Law is the least applicable to respiration compared to other gas laws like Boyle's Law and Dalton's Law because body temperature does not change much.
Charles's Law affects the volume of air we can inhale. When we breathe in cold air, it expands as it warms up in our lungs, leading to an increase in volume. This means we take shorter breaths in cold weather to account for the increased volume of air.







