Charles' Law states that the volume of a gas is directly proportional to its temperature. This law was first studied by French scientist Jacques Alexander Charles in 1787, who observed the effects of temperature on balloons. Charles' Law is relevant to scuba diving as it helps divers understand the impact of temperature changes on the pressure and volume of gas in their tanks. For example, if a scuba tank filled to capacity is left in the sun, the pressure inside the tank will increase, giving the false impression of more gas supply. Conversely, if a full tank is taken on a hot day and then submerged in cold water, the pressure will decrease, resulting in reduced gas supply. Understanding Charles' Law is crucial for scuba divers to ensure their safety and avoid running out of air during a dive.
What You'll Learn

Scuba tanks and temperature
When applied to scuba diving, Charles' Law helps explain the behaviour of gas inside a scuba tank when exposed to temperature variations. Scuba tanks are typically made of steel or aluminium and are filled with compressed air or other gas mixtures. The gas inside the tank is under pressure, and understanding how temperature affects this pressure is essential for safe diving.
Firstly, it's important to note that scuba tanks are non-flexible containers. According to Charles' Law, when the temperature of a non-flexible container increases, the pressure inside the container also increases while the volume remains constant. This principle can be observed when a scuba tank is left in the hot sun or in a hot car. The tank's internal pressure will rise, giving the false impression of a greater gas supply. Conversely, if a full tank is taken from a hot environment into cold water, the pressure will decrease, resulting in a reduced gas supply.
Additionally, the act of filling a scuba tank can also increase its temperature. As more gas is added to the fixed volume of the tank, the pressure and temperature rise. This is why scuba tanks often feel hot when being filled. It's crucial to allow the tank to cool down to ambient temperature before use, as the pressure will decrease as it cools. Dive shops often overfill tanks to compensate for this temperature-related pressure loss.
Moreover, Charles' Law highlights the importance of proper storage and handling of scuba tanks. Tanks should be stored in cool, shaded areas, as excessive heat can lead to increased pressure and, in rare cases, even cause the tank to explode. Modern tanks are equipped with safety plugs that will release pressure to prevent explosions.
In summary, Charles' Law helps divers understand the complex relationship between temperature and gas behaviour in scuba tanks. By recognising how temperature fluctuations impact pressure, divers can ensure they have an accurate understanding of their gas supply and take necessary precautions to avoid hazardous situations.
Criminal Liability and Respondeat Superior in Israeli Law
You may want to see also

Gas volume and temperature
Gases and their behaviour are crucial to understanding scuba diving, and Charles's Law is one of the fundamental principles that govern this. Jacques Charles, a French scientist, mathematician, inventor and balloonist, first studied the effects of temperature on gas volumes in 1787, and formulated what we now call Charles's Law.
Charles's Law states that the volume of a given mass of gas increases or decreases by the same factor as its temperature increases or decreases, assuming the pressure remains constant. In the context of scuba diving, this law is important for understanding the behaviour of gases in tanks and in dry suits.
When a scuba tank is filled with compressed air, the friction of the process heats up the air inside. As per Charles's Law, this increase in temperature leads to an increase in pressure inside the tank. This is why scuba tanks get hot when being filled. The pressure inside the tank will also be affected by the external temperature. For example, if a tank is left in the hot sun, the pressure inside will increase, giving the false impression of more gas supply. Conversely, if a tank is filled to capacity and then taken into cold water, the pressure will decrease, leading to a reduction in gas supply. This is a crucial consideration for divers, who must be aware of how temperature changes can impact their gas supply.
Dive shops often overfill air tanks to compensate for the potential decrease in gas volume due to colder water temperatures. Additionally, modern tanks have a safety plug that will release pressure before the tank explodes due to excessive heat. Proper storage of tanks is essential to avoid the dangers of overheating.
Charles's Law also has implications for divers using dry suits. A dry suit is a watertight garment that divers wear to stay warm by trapping a layer of air between their body and the suit. During a dive, divers can adjust the air volume in their suits to account for pressure changes during ascent and descent. However, if the air temperature is colder than the water temperature when they emerge, the gas volume in the suit can decrease, causing the suit to "vacuum seal" around them. This can be remedied by adding air to the suit or unzipping it to release the pressure.
Exploring Delivery Driver Privileges: Trespassing Law Exemptions
You may want to see also

Pressure and temperature
Charles's Law states that at a constant volume, the pressure of a gas varies directly with its absolute temperature. This law was formulated by French scientist Jacques Alexander Charles in 1787, who first studied the effects of temperature on the volume of a gas.
The law can be applied to the scenario of a scuba tank filled to 3000 PSI. If heated, the pressure in the tank will increase as the volume of the air inside remains constant. This is because the molecules of air inside the tank move more rapidly when heated, increasing their impact on the interior of the tank.
Charles's Law is relevant to scuba diving safety in several ways. Firstly, it highlights the dangers of leaving scuba tanks in the hot sun or in a hot car. The gas under pressure, when subjected to heat, can cause the tank to explode. Therefore, proper storage of air tanks is crucial to ensure they aren't left in direct sunlight. Additionally, the law explains why the pressure in a scuba tank may appear to drop or increase slightly depending on whether the tanks were filled outdoors or indoors. When a tank is filled rapidly, the temperature can rise, and as the tank cools down, the gas pressure decreases.
Charles's Law also explains why scuba tanks get hot when being filled with compressed air. The act of filling the tank involves adding gas to a fixed volume, which increases the temperature. Conversely, opening a tank valve and releasing air rapidly will decrease the temperature. This principle is important to understand when ice diving, as it explains why environmental seals are recommended in the regulator's first stages to prevent freezing.
Moreover, Charles's Law is applicable when considering the air temperature and water temperature during a dive. If the air temperature is colder than the water temperature, divers can become "vacuum-sealed" in their dry suits due to the decrease in gas volume. This can be mitigated by adding air to the suits from their tanks or unzipping the suits to release the pressure.
In summary, Charles's Law helps divers understand the effects of temperature changes on the pressure and volume of gas in their scuba tanks, contributing to their overall safety and understanding of the equipment.
Understanding Lemon Law Applicability for Private Owners
You may want to see also

Gas laws and scuba diving safety
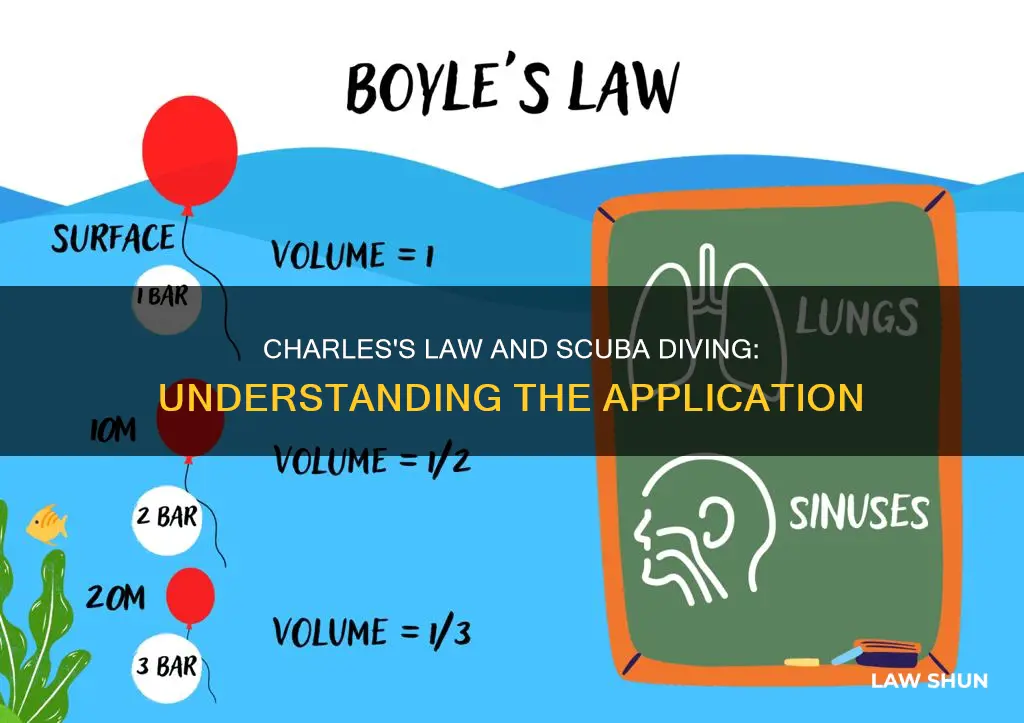
Scuba diving is an extreme sport that requires specialised training and equipment. One of the fundamental rules of scuba diving is to "never hold your breath". This is explained by Boyle's Law, which states that the pressure and volume of a gas are inversely proportional when the temperature is constant. As a diver descends, the pressure increases, and the volume of gas in their lungs decreases. Holding their breath while ascending can cause the lungs to expand to several times their normal volume, resulting in severe and possibly fatal damage.
Gay-Lussac's Law, also known as Amontons' Law, is another important principle in scuba diving. It states that the pressure of a gas is directly proportional to its temperature. This law explains why the pressure in a scuba tank increases when the temperature rises.
While Charles' Law is less relevant to diver safety, it is still important to understand. Charles' Law states that the volume of a gas is directly proportional to its temperature, provided the pressure remains constant. This law explains why scuba tanks should not be left in hot environments, as the pressure inside the tank will increase, potentially causing it to explode. It also explains why a tank filled with compressed air gets hot.
In addition, Charles' Law can help divers understand the phenomenon of "vacuum sealing" in dry suits. A dry suit is a watertight garment that divers wear to trap a layer of air and insulate themselves from cold water. When the air temperature is colder than the water temperature, the gas volume in the suit decreases, creating a "squeeze". Divers can adjust for this by adding air to their suits or unzipping them.
Overall, a basic understanding of gas laws is essential for ensuring safety in scuba diving.
Alabama's Three-Strike Law: Class C Felony Implications
You may want to see also

Charles' Law and scuba equipment
Charles's Law states that "the amount of change in either volume or pressure of a given gas volume is directly proportional to the change in the absolute temperature." This means that as the temperature of a gas increases, so does its volume, provided that the pressure remains constant. Conversely, if the temperature of a gas decreases, its volume will also decrease, as long as the pressure is kept constant.
Jacques Charles, a French scientist, mathematician, and inventor, first studied the effects of temperature on gas volumes and formulated Charles's Law in 1787. The law is expressed mathematically as:
> P1 x V1 / T1 = P2 x V2 / T2
Where P represents pressure, V volume, and T temperature. The subscripts 1 and 2 indicate the initial and final states of these variables, respectively.
Charles's Law is relevant to scuba diving because it helps explain the behaviour of gases in scuba tanks and how they are affected by temperature changes. When a scuba tank is filled with compressed air, the gas inside the tank is under pressure. If the tank is then left in a hot environment, such as in direct sunlight or in the trunk of a car, the pressure inside the tank will increase further due to the increase in temperature. This is in accordance with Charles's Law, as the volume of the tank remains constant.
Conversely, if a scuba tank is filled to capacity and then subjected to colder temperatures, the pressure inside the tank will decrease. This is important for divers to understand because it means that a tank filled to capacity in a warm environment may appear to have less gas volume when brought into a colder underwater environment. To compensate for this, dive shop owners often overfill air tanks to ensure divers have access to the expected amount of gas during their dives.
Additionally, Charles's Law highlights the dangers of storing filled scuba tanks in hot environments. As the temperature of the gas inside the tank increases, so does the pressure, which can lead to an increased risk of the tank exploding. Modern tanks have safety plugs that will release pressure before the tank explodes, but it is still crucial to store tanks properly and avoid leaving them in hot cars or boats.
While Charles's Law is not as directly applicable to diver safety as other gas laws, it helps divers understand the everyday phenomena they observe with their scuba equipment and gain a better grasp of the underlying principles of scuba diving.
Hiring Laws: Private Companies and Anti-Discrimination Compliance
You may want to see also
Frequently asked questions
Charles's Law states that the volume of a gas is directly proportional to its temperature. This means that as the temperature of a gas increases, so does its volume, and vice versa. This principle can be applied to the air in a scuba tank. For example, if a scuba tank is left out in the sun, the pressure inside the tank will increase, giving the false impression of more gas supply.
According to Charles's Law, an increase in temperature will lead to an increase in volume, assuming the pressure remains constant. However, since a scuba tank is a rigid container with a constant volume, an increase in temperature will result in an increase in pressure.
Filling a scuba tank with compressed air involves adding more gas molecules to the tank, which increases the pressure and temperature. This phenomenon can be explained by Charles's Law, as the volume of the tank remains constant while the temperature and pressure increase.
Scuba tanks should not be left in hot environments, such as in direct sunlight or in a hot car, as the increase in temperature can cause the pressure inside the tank to rise, potentially leading to an explosion. Proper storage ensures the safety of the equipment and the surrounding environment.
Dry suits are watertight garments that divers wear to stay warm by trapping a layer of air between the suit and the body. Charles's Law comes into play when the air temperature is colder than the water temperature. In such cases, the gas volume in the suit decreases, creating a "vacuum seal" effect. Divers can counteract this by adding air to the suit or unzipping it to release the pressure.







