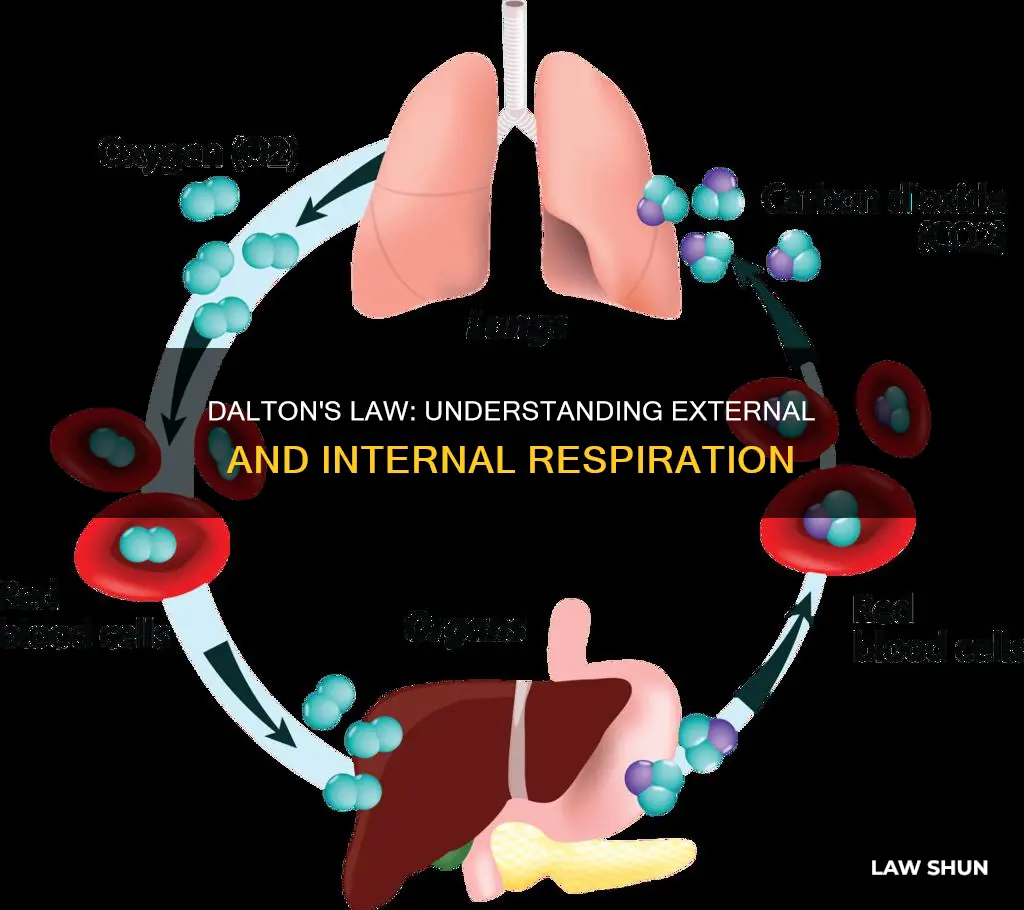
Dalton's Law of Partial Pressure, observed by John Dalton in 1801, states that the pressure of a mixture of gases is the sum of the pressures of the individual components. This law is fundamental in determining the behaviour of gases under different conditions, such as changes in temperature or volume. Dalton's law is particularly relevant to respiration, as it helps explain the mechanisms of gas exchange in the lungs. The law states that the total pressure exerted by a mixture of gases is the sum of the partial pressures of each gas in the mixture. In the context of respiration, this means that the pressure of atmospheric air is the sum of the partial pressures of its components, including nitrogen, oxygen, carbon dioxide, and other trace gases. During external respiration, which occurs in the alveoli of the lungs, oxygen enters the bloodstream, and carbon dioxide exits. The exchange of gases between the lungs and the blood is driven by partial pressure differences and is a passive process. Similarly, during internal respiration, which occurs in the tissues, there is a gas exchange between the blood and the tissues, with oxygen diffusing out of the blood and into the cells, and carbon dioxide diffusing out of the cells and into the blood.
What You'll Learn

Dalton's Law and the behaviour of gases
Dalton's Law of Partial Pressure, observed by John Dalton in 1801, states that the pressure of a mixture of gases is the sum of the pressures of the individual components. In other words, it is the average pressure exerted by all the gas particles in a given system. This law is fundamental in determining the behaviour of gases under different conditions, such as changes in temperature or volume.
Mathematically, the pressure of a mixture of gases can be defined as the sum of the partial pressures of each of the gases in the air.
> Ptotal=P1+P2+P3+⋯+Pn=n∑i=1Pi
In the context of atmospheric air, this can be expressed as:
> Atm=PN2+PO2+PCO2+PH2O+P(other gases)
Dalton's Law is particularly relevant to respiration, as it helps explain the process of external and internal respiration. External respiration refers to the exchange of gases between the lungs and blood, while internal respiration involves the exchange of gases between the blood and tissues. Both types of respiration are based on the diffusion of gases from high to low concentration. The best way to measure the concentration of gases is by measuring their partial pressure.
According to Dalton's Law, the total pressure exerted by a mixture of gases is the sum of the pressures exerted by each gas, and the partial pressure of each gas is directly proportional to its percentage in the mixture. This means that by increasing the percentage of any gas in a mixture, a higher partial pressure of that gas can be achieved. This principle is applied in oxygen therapy, where patients with diminished lung capacity are provided with air that has a higher concentration of oxygen, thereby increasing the pressure and amount of oxygen delivered to them.
Dalton's Law also explains the behaviour of gases in hyperbaric and hypobaric conditions. In hyperbaric conditions, such as underwater or in hyperbaric chambers, individual gas pressures increase as the pressure is higher than that of the atmosphere. In contrast, in hypobaric conditions, such as at high altitudes, individual gas pressures decrease but remain in proportion to their concentration in the mixture.
Hubble's Law: Universal or Group-Specific?
You may want to see also

External respiration
As the blood is pumped through this capillary network, gas exchange occurs. Although a small amount of oxygen is able to dissolve directly into the plasma from the alveoli, most of the oxygen is picked up by erythrocytes (red blood cells) and binds to a protein called hemoglobin. Oxygenated hemoglobin is red, causing the overall appearance of bright red oxygenated blood, which returns to the heart through the pulmonary veins. Carbon dioxide is released in the opposite direction of oxygen, from the blood to the alveoli. Some of the carbon dioxide is returned on hemoglobin, but it can also be dissolved in plasma or is present in a converted form.
The partial pressure of carbon dioxide is also different between the alveolar air and the blood of the capillary. However, the partial pressure difference is less than that of oxygen, at about 5 mm Hg. The partial pressure of carbon dioxide in the blood of the capillary is about 45 mm Hg, whereas its partial pressure in the alveoli is about 40 mm Hg. However, the solubility of carbon dioxide is much greater than that of oxygen—by a factor of about 20—in both blood and alveolar fluids. As a result, the relative concentrations of oxygen and carbon dioxide that diffuse across the respiratory membrane are similar.
Efficient external respiration depends on three things: partial pressure gradients and gas solubilities, ventilation-perfusion coupling, and the structural characteristics of the respiratory membrane.
Reflection Law: Universal or Surface-Specific?
You may want to see also

Internal respiration
The exchange of gases between the bloodstream and the tissues is influenced by several factors, including the partial pressure gradients between the blood and the tissues, the blood perfusion of those tissues, and the surface areas of those tissues. Oxygen diffuses from the blood into the cells of the tissues, while carbon dioxide diffuses out of the cells of the tissues and into the bloodstream.
Cellular respiration is the metabolic process by which an organism obtains energy through the reaction of oxygen with glucose. This process produces water, carbon dioxide, and adenosine triphosphate (ATP), which is the functional source of energy for the cell. The carbon dioxide produced during cellular respiration comes from the carbon in glucose and the oxygen used in the process.
The oxygen supply for cellular respiration comes from the external respiration of the respiratory system. External respiration is the exchange of gases that occurs in the alveoli of the lungs, where oxygen is picked up and carbon dioxide is released. The pulmonary artery carries deoxygenated blood into the lungs from the heart, where gas exchange occurs at the respiratory membrane—where the alveolar and capillary walls meet. Although a small amount of oxygen dissolves directly into the plasma from the alveoli, most of it is picked up by red blood cells and binds to a protein called hemoglobin.
During internal respiration, the partial pressure gradients are opposite to those present during external respiration. The partial pressure of oxygen in the tissues is low (around 40 mm Hg) due to continuous use for cellular respiration, while the partial pressure of oxygen in the blood is about 100 mm Hg. This pressure gradient causes oxygen to dissociate from hemoglobin, diffuse out of the blood, cross the interstitial space, and enter the tissue.
As cellular respiration continuously produces carbon dioxide, its partial pressure is lower in the blood than in the tissue. This causes carbon dioxide to diffuse out of the tissue, cross the interstitial fluid, and enter the blood. The blood then carries the carbon dioxide back to the lungs, either bound to hemoglobin, dissolved in plasma, or in a converted form, to be exhaled from the body.
Romeo and Juliet Law: Texas' Take on Young Love
You may want to see also

Partial pressure and its role in respiration
Dalton's Law of Partial Pressure, observed by John Dalton in 1801, states that the pressure of a mixture of gases is the sum of the pressures of the individual components. In other words, it is the average pressure exerted by all the gas particles in a given system.
The air in the atmosphere is a mixture of many different gases, primarily nitrogen, oxygen, water vapour, and carbon dioxide. Each of these gases exerts a pressure known as its partial pressure. The total pressure exerted by the mixture of gases is the sum of the partial pressures of each gas in the mixture. This law is fundamental in determining the behaviour of gases under different conditions, such as changes in temperature or volume.
Partial pressure is extremely important in predicting the movement of gases. Gases tend to equalise their pressure in two regions that are connected. A gas will move from an area where its partial pressure is higher to an area where its partial pressure is lower. The greater the partial pressure difference, the more rapid the movement of gases.
During respiration, gas exchange occurs at two sites in the body: in the lungs, where oxygen is picked up and carbon dioxide is released at the respiratory membrane, and at the tissues, where oxygen is released and carbon dioxide is picked up. External respiration is the exchange of gases with the external environment and occurs in the alveoli of the lungs. Internal respiration is the exchange of gases with the internal environment and occurs in the tissues. The actual exchange of gases occurs due to simple diffusion. Energy is not required to move oxygen or carbon dioxide across membranes. Instead, these gases follow pressure gradients that allow them to diffuse.
The partial pressure of oxygen in the alveoli is about 104 mm Hg, whereas the partial pressure of the oxygenated pulmonary venous blood is about 100 mm Hg. The partial pressure of carbon dioxide in the blood of the capillary is about 45 mm Hg, whereas its partial pressure in the alveoli is about 40 mm Hg. The solubility of carbon dioxide is much greater than that of oxygen in both blood and alveolar fluids. As a result, the relative concentrations of oxygen and carbon dioxide that diffuse across the respiratory membrane are similar.
In the tissues, the partial pressure of oxygen is low, about 40 mm Hg, because oxygen is continuously used for cellular respiration. In contrast, the partial pressure of oxygen in the blood is about 100 mm Hg. This creates a pressure gradient that causes oxygen to dissociate from haemoglobin, diffuse out of the blood, cross the interstitial space, and enter the tissue. Considering that cellular respiration continuously produces carbon dioxide, the partial pressure of carbon dioxide is lower in the blood than it is in the tissue, causing carbon dioxide to diffuse out of the tissue, cross the interstitial fluid, and enter the blood.
Jim Crow Laws: Impact on Asian Americans
You may want to see also

Dalton's Law in hyperbaric conditions
Dalton's Law of Partial Pressure, observed by John Dalton in 1801, states that the pressure of a mixture of gases is the sum of the pressures of the individual components. This law is fundamental in determining the behaviour of gases under different conditions, such as changes in temperature or volume.
In the context of respiration, Dalton's law has important implications for both external and internal respiration. During external respiration, we breathe atmospheric air, which is a mixture of gases, including nitrogen, oxygen, carbon dioxide, and water vapour. According to Dalton's law, the total atmospheric pressure is the sum of the partial pressures of each of these gas components. This law also implies that the relative concentration of gases remains constant even as the pressure and volume of the gas mixture change. Therefore, the air we inhale into our lungs will have the same relative concentration of gases as the atmospheric air.
In the lungs, the relative concentration of gases, particularly oxygen and carbon dioxide, is crucial for gas exchange. Dalton's law helps explain why oxygen moves into the alveoli while carbon dioxide moves out. This is because gases flow from areas of high pressure to low pressure. Atmospheric air has a higher partial pressure of oxygen than the air in the alveoli, so oxygen moves into the alveoli. Conversely, the partial pressure of carbon dioxide is higher in the alveoli than in atmospheric air, leading to the diffusion of carbon dioxide out of the alveoli.
Now, let's discuss the application of Dalton's Law in hyperbaric conditions:
Hyperbaric chambers are often used in medical settings to treat various conditions, such as burns, certain cancers, and carbon monoxide poisoning. These chambers are pressurised to create an environment with higher atmospheric pressure than normal. This increase in pressure leads to a higher concentration of oxygen dissolved in the blood, as per Dalton's Law. At higher pressures, the partial pressure of oxygen increases, resulting in a higher concentration of oxygen dissolved in the blood. This increase in oxygen concentration can be beneficial for treating certain conditions.
The use of hyperbaric chambers is also relevant in treating scuba divers suffering from decompression sickness, commonly known as the bends. When a diver ascends too quickly, the nitrogen dissolved in their blood forms bubbles that can block blood flow. By placing the diver in a hyperbaric chamber and gradually decreasing the pressure, the nitrogen slowly diffuses out through the lungs. This application of hyperbaric conditions and Dalton's Law helps to safely reduce the pressure and prevent further complications.
It is important to note that Dalton's Law is most accurate for ideal gases and may not hold completely true for all gases, especially under extremely high-pressure conditions or when intermolecular forces are at play.
Libel Law: Aggregated Stories' Legal Liability
You may want to see also
Frequently asked questions
Dalton's law of partial pressure states that the pressure of a mixture of gases is the sum of the pressures of the individual components.
External respiration is the exchange of gases with the external environment, which occurs in the alveoli of the lungs. Dalton's law applies to external respiration as it explains the behaviour of gases during the exchange of gases. It states that each specific gas in a mixture exerts its own pressure, and the total pressure exerted by a mixture of gases is the sum of the partial pressures of the gases in the mixture.
Internal respiration is the exchange of gases with the internal environment, which occurs in the tissues. Dalton's law applies to internal respiration as it explains the movement of gases from an area of high pressure to an area of low pressure. It also explains how gases dissolve in the blood.
Dalton's law is only completely accurate for ideal gases. It may not hold true in conditions of extremely high pressure or when intermolecular forces are at play.







