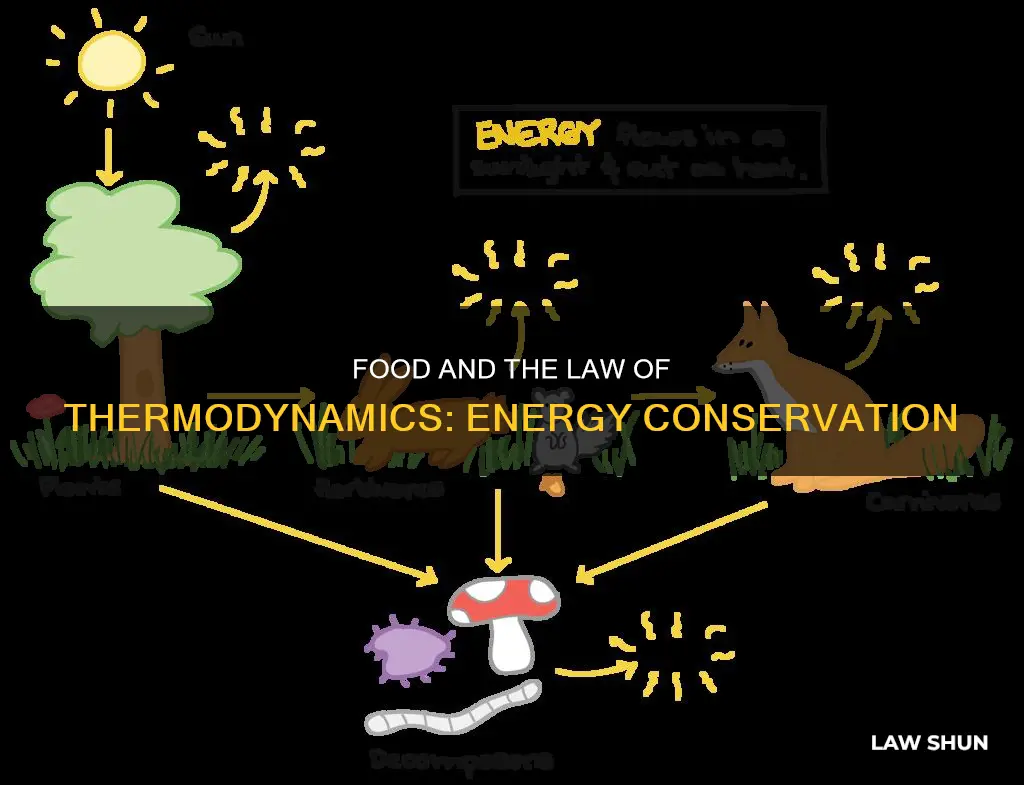
The laws of thermodynamics are fundamental principles in modern physics, outlining how temperature, energy, and entropy behave under different conditions. The first law of thermodynamics, also known as the law of energy conservation, states that energy cannot be created or destroyed, only transformed. This is evident in the process of photosynthesis, where plants convert light energy from the sun into chemical energy stored as complex carbohydrates. Animals then consume these plants, and during digestion, sugars are broken down to release energy as heat or provide chemical energy. This process adheres to the first law, as energy is neither created nor destroyed but changes form.
However, the second law of thermodynamics introduces the concept of entropy, which measures the randomness or disorder within a closed or isolated system. As usable energy decreases and unusable energy increases, entropy increases, leading to a progression towards disorder that cannot be reversed. This law suggests that our universe is becoming increasingly disordered, and all actions contribute to this degeneration.
While these laws govern the behaviour of energy in various systems, including biological processes, the question arises: how does food fit into these laws without breaking them?
What You'll Learn

The First Law of Thermodynamics
In the context of food, the First Law of Thermodynamics can be observed in a classic food web where plants harness light energy from the sun and convert it into chemical energy, storing it as complex carbohydrates through photosynthesis. This process releases oxygen into the atmosphere, and the plants eventually become food for animals. During digestion, animals break down the sugars from the plants, releasing energy as heat or as chemical energy from glucose to drive cellular processes for survival and reproduction. This energy can also be stored in macromolecules like glycogen and fatty acids, which serve as energy reserves that can be quickly accessed during times of high energy demand.
The law also plays a role in understanding the inefficiencies in energy transfer within food chains. According to the Second Law of Thermodynamics, as usable energy within a closed or isolated system decreases and unusable energy increases, entropy or disorder also increases. In a food chain, this means that energy is lost as heat between trophic levels, with consumers gaining only a fraction of the energy stored in their food. This inefficiency in energy transfer has important implications for understanding the dynamics of biological systems and the flow of energy through different trophic levels.
Racial Profiling: Is It Legal?
You may want to see also

The Second Law of Thermodynamics
In the context of food, the Second Law of Thermodynamics is evident in the food web, where plants convert radiant energy from the sun into chemical energy, which is stored as complex carbohydrates. When these plants are consumed by animals, the sugars from the carbohydrates are broken down during digestion, releasing energy as heat. This energy can also be used to drive cellular processes or stored as a reserve for future use. However, the energy available to consumers at each trophic level in the food chain is less than the energy stored in their food source, as some energy is always lost as heat. This loss of energy between trophic levels demonstrates the Second Law of Thermodynamics in action, as it represents the transition of energy from usable to unusable forms.
The law also relates to the concept of entropy, which is a measure of randomness or disorder within a closed or isolated system. In the context of food and biology, the energy required to maintain the complex structure of a cell increases entropy in the outside environment. This increase in entropy contributes to the overall increase in disorder and inefficiency described by the Second Law of Thermodynamics.
While the Second Law of Thermodynamics suggests a bleak and desolate fate for the universe, it is important to note that researchers have recently discovered loopholes that may allow for the creation of systems where entropy decreases, at least on a microscopic scale and in the short term. These findings could have significant implications for our understanding of the law and the potential for local quantum perpetual motion machines.
Betting Scandal: Pete Rose's Actions and Legal Consequences
You may want to see also

Energy transfer in food chains
The first law of thermodynamics states that the total amount of energy in the universe is constant and conserved. In other words, there has always been, and always will be, the same amount of energy in the universe. Energy can be transferred or transformed but not created or destroyed.
Energy exists in many forms, including light energy, kinetic energy, heat energy, potential energy, and chemical energy. For example, plants convert light energy from the sun into chemical energy stored within biological molecules through photosynthesis. This chemical energy provides living cells with energy from food.
A food chain is a linear sequence of organisms through which energy passes as they eat and are eaten. Each organism in a food chain occupies a specific trophic level, or energy level. The first trophic level consists of producers, such as plants, which convert solar energy into chemical energy through photosynthesis. The second trophic level is made up of primary consumers (herbivores) that eat the producers. Higher-level consumers, including secondary and tertiary consumers, are carnivores or omnivores that eat other animals, completing the energy transfer through the food chain.
However, the second law of thermodynamics states that not all energy transfers and transformations are completely efficient. In every energy transfer, some amount of energy is lost as heat. For example, in a food chain, only about 10% of the energy in one trophic level is passed on to the next level. This loss of energy as heat is in accordance with the second law of thermodynamics, which states that energy losses in a system lead to an increase in entropy, or disorder. Thus, the energy transfer in food chains, with their associated energy losses, does not break the laws of thermodynamics but instead aligns with them.
Consequences of Breaching Attorney-Client Privilege Law
You may want to see also

Energy release as heat
The First Law of Thermodynamics states that energy cannot be created or destroyed, only transformed. This is also known as the principle of conservation of energy. It is one of the most fundamental laws of physics, governing everything from the heat required to warm up a cup of coffee to the chemical reactions that produce oxygen in the leaves of trees.
The law of conservation of energy applies to the digestion of food. During digestion, sugars are broken down and energy is released as heat. This is an example of energy transformation. The energy in the food is converted into heat energy, which is then used by the body to perform various functions, such as maintaining body temperature.
The human body is an open system, which means it can exchange energy with its surroundings. During the process of digestion, some energy is released as heat, which increases the entropy of the universe. Entropy is a measure of randomness or disorder in a system. As energy is transferred or transformed, the universe's entropy increases.
The release of energy as heat during digestion is a crucial process for maintaining the body's temperature and supporting various metabolic processes. This energy transformation demonstrates the First Law of Thermodynamics in action, as the energy from the food is not destroyed but transformed into a form that the body can utilize.
In summary, the digestion of food does not break the First Law of Thermodynamics because the energy in the food is not destroyed. Instead, it is transformed and released as heat, contributing to the body's overall energy balance and supporting various physiological functions. This energy transformation is a fundamental aspect of human metabolism and highlights the importance of thermodynamic principles in understanding biological systems.
McCabe's Actions: Lawful or Unlawful?
You may want to see also

The role of decomposers
Decomposers are the final link in the food chain. They are the "clean-up crew" or "housekeepers" of an ecosystem, breaking down dead plants and animals and returning vital nutrients to the soil. Without decomposers, dead animal carcasses would pile up, and the soil would lack nutrients, causing the entire ecosystem to break down.
Decomposers are made up of the FBI—fungi, bacteria, and invertebrates such as worms and insects. They are all living things that get energy by eating dead animals and plants and breaking down wastes of other animals.
Fungi are the main decomposers in many ecosystems, particularly forests. They help release nitrogen and phosphorus from dead decaying matter through a series of specialized proteins and enzymes in their cell walls and hyphae (root-like filaments). Fungi are particularly suited to penetrating large pieces of decaying matter like wood with their hyphae and breaking it down with lignin-digesting enzymes.
Bacteria are also key decomposers. They are critical in the early stages of decomposition before fungi and earthworms take over. Bacteria not only feed on dead leaves and weeds but they also fix nitrogen in the soil so it is not lost to the air or water. Bacteria transform nitrogen into a form that can be used by other organisms in the food chain. In some plants like legumes, the bacterium Rizobium lives in nodules on the roots of the plants in a symbiotic relationship. In turn for giving them a place to live, the bacteria return the favor by fixing nitrogen for the plants to use.
Other organisms that break down decaying material include earthworms, some insects, sea cucumbers, and woodlice. These organisms are called detritivores as they need to ingest the material first, unlike fungi that use chemical and biological processes.
Decomposers are involved in virtually all of the nutrient cycles on the planet. They are responsible for returning nutrients from dead organic matter back into the soil, which is then used by plants for photosynthesis. This gives the food chain its cyclical nature.
Protesters' Rights: Street Standing and the Law
You may want to see also
Frequently asked questions
The first law of thermodynamics states that energy cannot be created or destroyed, only transformed. This is demonstrated in a classic food web where light energy from the sun is converted into radiant energy by plants, then into chemical energy, and stored as complex carbohydrates. The plants are then consumed by animals, and during the digestion process, the sugars release energy as heat.
The second law of thermodynamics states that as usable energy within a closed or isolated system decreases, and unusable energy increases, entropy also increases. In food chains, energy escapes as heat between trophic levels, with consumers gaining only a small percentage of the energy stored in their food.
The third law of thermodynamics states that a system's entropy tends to increase over time, and as it approaches absolute zero, the entropy approaches a minimum value. Food does not break this law as it is in line with the natural increase in entropy over time.







