The law of conservation of mass, also known as the principle of mass conservation, states that mass within a closed system remains the same over time. In other words, mass can neither be created nor destroyed, only changed in form. This means that the total mass of all substances before a chemical reaction will equal the total mass of all substances after a chemical reaction. This principle is widely used in many fields, including chemistry, mechanics, and fluid dynamics.
| Characteristics | Values |
|---|---|
| Definition | The law of conservation of mass states that mass within a closed system remains the same over time. |
| Discovery | Antoine Laurent Lavoisier discovered the law of conservation of mass in 1789. |
| Formula | The law can be expressed in differential form using the continuity equation in fluid mechanics and continuum mechanics. |
| Application | The concept of mass conservation is widely used in many fields such as chemistry, mechanics, and fluid dynamics. |
| Limitations | The law of conservation of mass only holds approximately and is considered part of a series of assumptions in classical mechanics. |
What You'll Learn

Mass cannot be created or destroyed
The law of conservation of mass states that mass cannot be created or destroyed. This principle, also known as Lavoisier's law, was discovered by French chemist Antoine Lavoisier in 1789. It holds that the mass of an object or collection of objects remains constant, regardless of how the constituent parts rearrange themselves.
In other words, the amount or mass of matter in an isolated system will always remain the same, regardless of any chemical reactions or physical changes that take place. This is because mass is simply changing form. For example, when a liquid turns into a gas, the matter (the liquid) has not vanished. It has simply transformed into another substance of equal mass.
The law of conservation of mass is of great importance in chemistry, as it allows scientists to combine different materials and test the reactions between them. It is also used in many other fields, including mechanics and fluid dynamics.
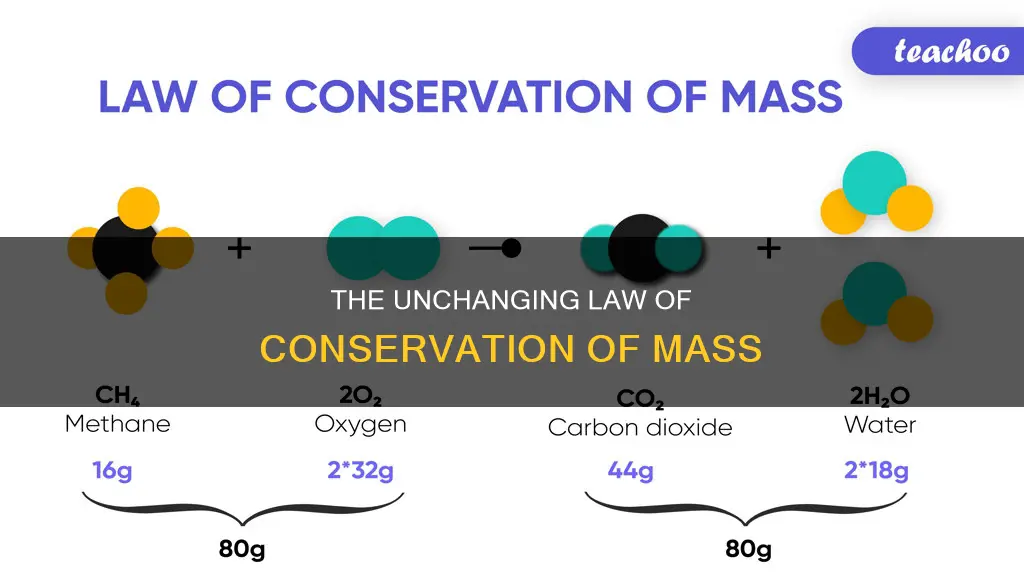
The concept of mass conservation is particularly useful when studying chemical reactions. For example, in the chemical reaction where one molecule of methane (CH4) and two oxygen molecules (O2) are converted into one molecule of carbon dioxide (CO2) and two of water (H2O), the number of molecules resulting from the reaction can be derived from the principle of conservation of mass. Initially, there are four hydrogen atoms, four oxygen atoms, and one carbon atom. Thus, the number of water molecules produced must be exactly two per molecule of carbon dioxide produced.
The law of conservation of mass also applies to everyday scenarios. For instance, when you light a candle, some of the wax is transformed into gases, namely water vapour and carbon dioxide. However, no matter (and therefore no mass) is lost through the process of burning.
Mendelian Inheritance: Multifactorial Traits Explained
You may want to see also

Mass can be transformed from one form to another
The law of conservation of mass states that mass within a closed system remains constant over time. In other words, mass cannot be created or destroyed in an isolated system. However, this law also implies that mass can be transformed from one form to another.
This concept is best illustrated through chemical reactions. For example, consider the reaction between silver nitrate and sodium chloride. When these two compounds dissolve in water, they form silver chloride and sodium nitrate. Silver chloride does not dissolve in water, so it forms a solid precipitate that can be filtered out. By evaporating the water, we can recover the sodium nitrate.
Now, let's apply the law of conservation of mass to this reaction. Suppose we start with 58.5 grams of sodium chloride and 169.9 grams of silver nitrate, giving us a total of 228.4 grams of reactants. After the reaction is complete and the products are separated, we find that we have formed 143.4 grams of silver chloride and 85.0 grams of sodium nitrate. The total mass of the products is still 228.4 grams, which is equal to the total mass of the reactants.
This example demonstrates that while the total mass remains the same, the individual substances involved in the reaction have transformed into different forms. The reactants, sodium chloride and silver nitrate, have changed into the products, silver chloride and sodium nitrate. The law of conservation of mass tells us that the mass of the reactants must always be equal to the mass of the products in any chemical reaction.
The concept of mass conservation is not limited to chemistry; it is also applicable in other fields such as mechanics and fluid dynamics. It plays a crucial role in solving engineering problems, where understanding the mass distribution of a system over time is essential.
HIPAA Compliance: COVID-19's Impact on Healthcare Privacy
You may want to see also

Mass is conserved in chemical reactions
The law of conservation of mass, also known as the principle of mass conservation, states that mass within a closed system remains constant over time. In other words, mass cannot be created or destroyed, only transformed from one form to another. This law applies to any system closed to all transfers of matter, meaning that the mass of the system as a whole remains the same.
This law has its roots in ancient Greek philosophy, with the idea that "nothing comes from nothing", and so what exists now has always existed, and no new matter can come into existence. This idea was further developed by Epicurus in the 3rd century BCE, who stated that "the totality of things was always such as it is now, and always will be".
In the context of chemical reactions, the law of conservation of mass dictates that the total mass of the products must be equal to the total mass of the reactants. This means that during a chemical reaction, mass is conserved, and there is no overall change in mass. For example, in the reaction between silver nitrate and sodium chloride, the total mass of the products (silver chloride and sodium nitrate) is equal to the total mass of the reactants.
The concept of mass conservation is crucial in chemistry, as it forms the basis for stoichiometry, which is the calculation of the amount of reactant and products in a chemical reaction. By understanding that mass is conserved in chemical reactions, chemists can quantitatively study the transformations of substances, leading to the development of modern chemistry.
Castle Law: Family Violence Immunity?
You may want to see also

Mass is conserved in combustion processes
The law of conservation of mass, also known as the principle of mass conservation, states that mass within a closed system remains constant over time. In other words, mass can neither be created nor destroyed, only transformed from one form to another. This means that the mass of the reactants must be equal to the mass of the products.
The concept of mass conservation is particularly relevant to combustion processes. Combustion involves the conversion of energy and mass. For example, when burning wood, the reactants (wood, oxygen) are transformed into products (carbon dioxide, water vapour, and ash). Despite the apparent change in mass, the law of conservation of mass holds true. The mass of the wood before combustion is equal to the total mass of the products after combustion.
This principle can be applied to various combustion processes, such as the burning of hydrocarbons. For instance, in the combustion of hexanes, the mass of the reactants (hexanes and oxygen) is equal to the mass of the products (carbon dioxide, carbon monoxide, and water). This can be observed through controlled experiments where the masses of the reactants and products are precisely measured.
While mass is conserved in combustion processes, it is important to note that this conservation is not absolute. The law of conservation of mass is an approximation, and in reality, mass is not always perfectly conserved. This is because a small portion of mass can be converted into energy, as described by Einstein's equation, E=mc^2. However, the amount of mass converted is extremely negligible, and for practical purposes, we can assume that mass remains conserved in chemical reactions.
Mendel's Law of Inheritance and Corn: A Study
You may want to see also

Mass is conserved in open systems
The law of conservation of mass states that mass within a closed system remains constant over time. This means that mass can neither be created nor destroyed in a closed system but can be transformed from one form to another. For example, in a chemical reaction, the mass of the reactants must be equal to the mass of the products.
However, mass is not generally conserved in open systems, where energy or matter is allowed to enter or exit the system. In such cases, the law of conservation of mass does not strictly apply, and the total mass of the system can change.
For instance, consider boiling water on a stove. If you put a lid on the pot, the water vapour will collect inside, and the amount of mass will not change, though it will be converted from liquid to gas. This is a closed system. If you take the lid off, the evaporated water will escape into the room, and the total amount of matter will change. By removing the lid, you have created an open system.
The law of conservation of mass can be expressed mathematically using the continuity equation in fluid mechanics and continuum mechanics:
\(\begin{array}{l}\frac{\partial \rho }{\partial t}+\bigtriangledown (\rho v)=0\end{array} \)
▽ is the divergence
Understanding Chukim and Mishpatim: Jewish Dietary Laws Explained
You may want to see also
Frequently asked questions
The law of conservation of mass states that the mass of an object or collection of objects never changes, no matter how the constituent parts rearrange themselves.
The law of conservation of mass states that the mass of the reactants must be equal to the mass of the products for a low-energy thermodynamic process. In other words, mass is conserved in chemical reactions.
The law of conservation of mass does not apply to nuclear reactions. In nuclear reactions, there is a conversion between rest mass and energy, and the products may have a smaller or greater mass than the reactants.
Mass is not generally conserved in open systems. In an open system, energy or matter is allowed into or out of the system.
In systems with large gravitational fields, general relativity comes into play, and mass-energy conservation becomes a more complex concept. Neither mass nor energy is as strictly conserved as in special relativity.







