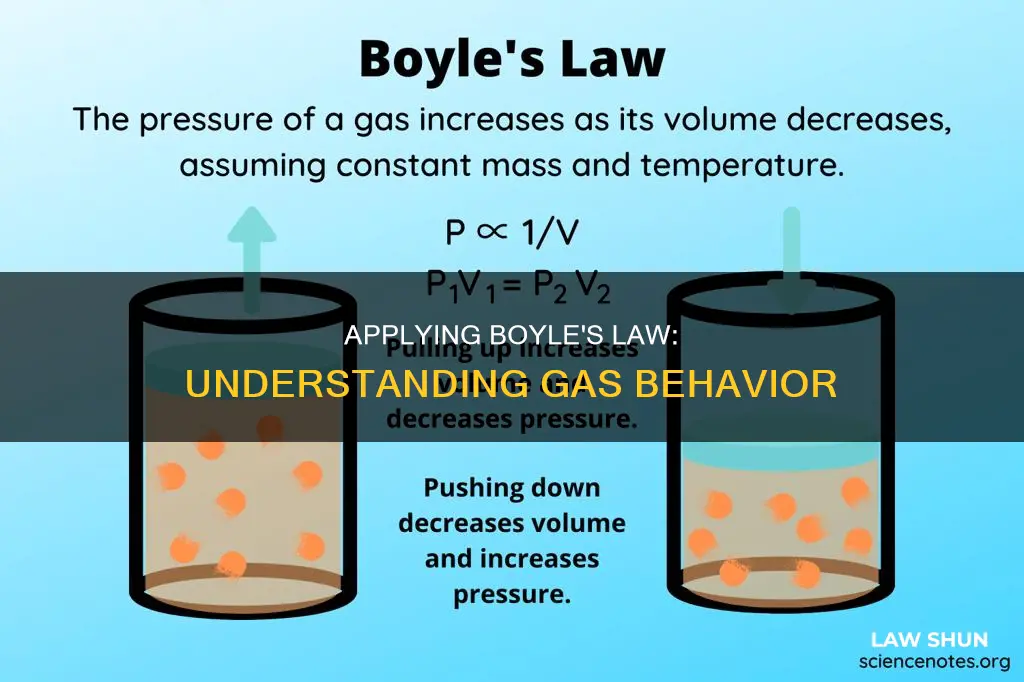
Boyle's Law, also known as the Boyle-Mariotte law, is an empirical gas law that describes the relationship between the pressure and volume of a confined gas. It states that when the temperature of a given mass of confined gas is constant, the product of its pressure and volume is also constant. In other words, the volume of a mass of gas is inversely proportional to its pressure. This means that if the volume increases, the pressure decreases, and vice versa. Boyle's Law can be expressed mathematically as PV=k, where P denotes pressure, V denotes volume, and k is a constant value representing the temperature of the system and amount of gas. This law has various real-life applications, such as in spray paint cans, syringes, and scuba diving.
What You'll Learn

Bicycle pumps
A bicycle pump works by pushing down on a piston, which decreases the volume of gas inside the pump. This decrease in volume leads to an increase in pressure, as the gas molecules have more collisions with the interior walls of the pump. This increase in pressure forces the air into the tyre.
When you pull the handle of the pump, the volume inside the cylinder increases, leading to a decrease in pressure. This decrease in pressure causes air to be drawn into the pump from the external environment.
The process of extracting air and then pumping it into the tyre is repeated until the tyre is completely filled. This simple mechanism is a practical application of Boyle's Law, demonstrating the relationship between pressure and volume of a confined gas.
Boyle's Law can be applied to various other real-life examples, such as the mechanics of human breathing and scuba diving. It is one of the fundamental gas laws that help us understand the behaviour of gases and their response to changes in pressure and volume.
Minimum Wage Laws: Rights for Undocumented Workers?
You may want to see also

Spray paints
Spray paint or aerosol spray is a classic example of Boyle's Law in action.
A spray paint can contains two substances: the paint itself, and a pressurised gas in liquid form. This gas has a boiling point below room temperature, but because the can is sealed, it doesn't boil and turn into a gas.
When you press the nozzle, the seal is broken, and the gas starts to boil and expand. This creates pressure inside the can, which pushes the paint out of the nozzle. The gas is trying to reach an area of lower pressure.
The spray paint can must be shaken before use. This is to ensure the paint and gas are thoroughly mixed. Inside the can, a ball bearing rattles around, which helps to mix the two substances.
The gas is compressed so much that it stays in a liquid state, even when heated past its boiling point. This is another example of Boyle's Law.
Boyle's Law states that when the temperature and concentration of a gas are kept constant, the pressure exerted by the gas is inversely proportional to the volume it occupies. In other words, as the volume of a gas increases, its pressure decreases, and vice versa. This is why the gas in a spray paint can expands and pushes the paint out when the pressure is released by opening the nozzle.
HIPAA Laws and Spouses: What You Need to Know
You may want to see also

Syringes
Boyle's Law, discovered by Robert Boyle in 1662, states that the pressure of a fixed amount of gas is inversely proportional to its volume at a constant temperature. In other words, if the volume of a gas increases, its pressure decreases, and vice versa. This principle can be applied to the operation of a syringe.
When the plunger of a syringe is pulled out, the volume inside the barrel increases, resulting in a decrease in pressure. This is because the gas inside the syringe now has more space to occupy, so its particles spread out and the pressure reduces. Conversely, when the plunger is pushed back in, the volume decreases and the pressure increases. The gas particles are forced closer together, increasing the pressure.
This principle can be observed in a simple experiment using a syringe. First, seal the tip of the syringe with a cap to isolate the air inside from the atmosphere. Pull the plunger upwards and note the volume reading. Then, place weights (such as books) on the plunger to push it downwards, compressing the air inside. By recording the weights of the books and the volume reading, you can establish the pressure-volume relationship. As more weight is added, the volume of air inside the syringe decreases, and the pressure increases.
Boyle's Law can also be applied to other examples, such as the operation of the lungs during breathing. When we inhale, our diaphragm lowers, increasing the volume inside our lungs and decreasing the pressure. This allows outside air to be drawn into our lungs. When we exhale, our diaphragm pushes upwards, reducing the volume inside our lungs and increasing the pressure, which forces the air out.
Lemon Law and Mobile Homes: What's the Verdict?
You may want to see also

Human breathing
Boyle's law states that the volume of a gas and pressure are inversely proportional to each other, provided the temperature and the amount of gas remain constant. This law helps us understand the relationship between pressure and volume in the lungs when breathing.
During inhalation, the lungs expand, causing the volume inside the lungs to increase, and the pressure inside to decrease. This follows Boyle's law, which states that as the volume increases, the pressure decreases. As the pressure inside the lungs is now lower than the pressure outside the body, air moves into the lungs. This process is known as inhalation.
During exhalation, the volume inside the lungs decreases, and the pressure increases. As a result, air moves out of the lungs.
The lungs do not follow Boyle's law at all volumes. For example, at low lung volumes, it takes a large change in pressure to make small changes in volume. At high volumes within the lung, it takes more negative pressure to expand the tissue.
Boyle's law also applies to other scenarios, such as the use of a syringe. When the plunger of the syringe is pulled, the volume inside the tube increases, and the pressure is reduced, causing external fluid to be drawn into the tube.
Antique Firearms: Concealed Carry Law Exemptions?
You may want to see also

Scuba diving
Boyle's Law, discovered by Robert Boyle in 1662, is a fundamental concept in physics that describes the relationship between the pressure and volume of a confined gas. It states that, at a constant temperature, pressure and volume are inversely proportional; as one increases, the other decreases. This principle has numerous applications in scuba diving, where divers encounter significant changes in pressure as they descend and ascend underwater.
When a scuba diver descends, the water pressure around them increases, leading to a decrease in the volume of the air in their scuba equipment and body according to Boyle's Law. This compression of air can cause discomfort and even injury if not properly managed. For example, divers must equalize the pressure in their ears to avoid pain and potential ear injuries, such as ear barotrauma. Similarly, they may need to adjust their buoyancy compensator device (BCD) to prevent loss of control during ascent.
Boyle's Law also plays a crucial role in understanding the safety guidelines for scuba diving. One of the most important rules is to never hold your breath underwater. If a diver ascends while holding their breath, the air in their lungs will expand as the surrounding pressure decreases, which can lead to pulmonary barotrauma. Additionally, ascending too quickly can result in decompression sickness. This is because the nitrogen gas absorbed by the diver's body during the dive expands rapidly as they ascend, and slow ascent allows the body to safely eliminate this nitrogen.
Furthermore, Boyle's Law helps explain the behaviour of air during a dive. As a diver descends, the air in their lungs, mask, ears, and sinuses is compressed due to the increased pressure. Conversely, when they ascend, the pressure decreases, and the air in these spaces expands. This expansion and compression of air can be observed in the scuba equipment as well, affecting the buoyancy of the diver.
It is important to note that Boyle's Law only applies at a constant temperature. Changes in temperature will impact the volume of gas, with heating causing it to expand and cooling causing it to compress. Therefore, when applying Boyle's Law in scuba diving, it is crucial to consider the surrounding water temperature and its potential impact on the gas volume.
Stark Law: Implications for Employed Physicians and their Employers
You may want to see also
Frequently asked questions
Boyle's Law is an empirical gas law that describes the relationship between the pressure and volume of a confined gas. It states that when the temperature is held constant, the volume of a given mass of gas is inversely proportional to its pressure.
The equation for Boyle's Law is: PiVi = PfVf, where Pi = initial pressure, Vi = initial volume, Pf = final pressure, and Vf = final volume.
First, identify the "given" information and what the problem is asking you to find. Then, rearrange the equation algebraically to solve for the unknown variable. Substitute the known quantities into the equation and solve.
Boyle's Law is applied when filling a tire with air. As you put more air into the tire, you are forcing the gas molecules together, reducing their volume and increasing the pressure. Another example is using a syringe. When you pull the plunger out, you increase the volume within the chamber, which causes a decrease in pressure and creates a vacuum, sucking fluid into the syringe.
Yes, although Boyle's Law is a special case of the ideal gas law, it can be applied to real gases at normal temperatures and low (ordinary) pressures.