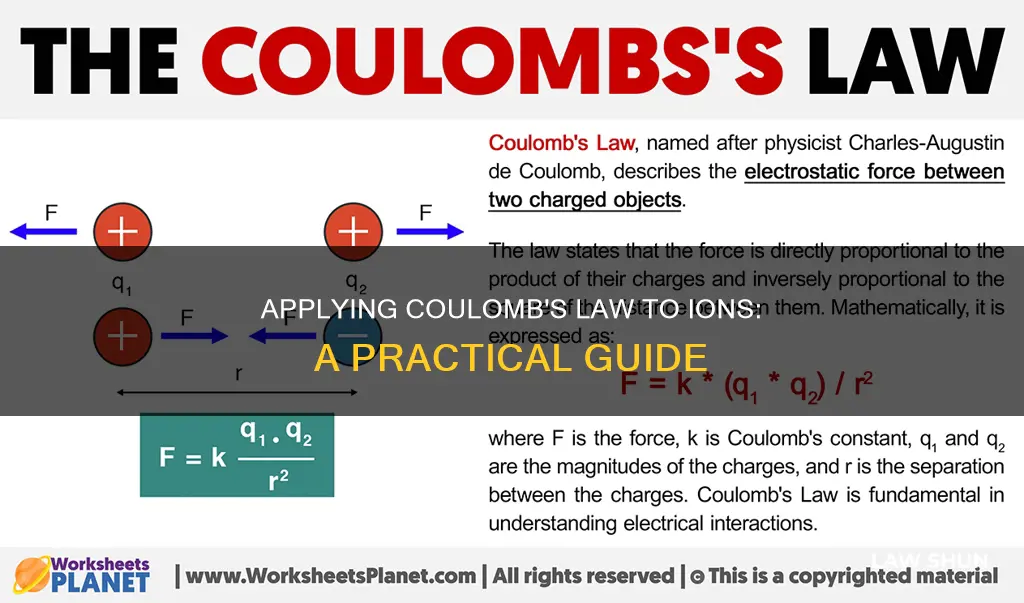
Coulomb's Law is a fundamental principle in physics that describes the electrostatic force between two charged particles at rest. It was first published in 1785 by French physicist Charles-Augustin de Coulomb, although the law was known earlier. Coulomb's Law states that the magnitude of the attractive or repulsive force between two charged particles is directly proportional to the product of their charges and inversely proportional to the square of the distance between them. This law is often referred to as an inverse-square law, as the force decreases as the distance between the particles increases. The formula for Coulomb's Law is given as:
> |F| = ke * (|q1| * |q2|) / r^2
where ke is Coulomb's constant, q1 and q2 are the charges of the particles, and r is the distance between them. Coulomb's Law is similar to Newton's law of universal gravitation but differs in the existence of two types of electric charge and the strength of the force. Coulomb's Law can be applied to a mass of ions to understand the electrostatic interactions and forces between them.
| Characteristics | Values | ||||||
|---|---|---|---|---|---|---|---|
| What is Coulomb's Law? | A mathematical formula that describes the electrostatic interaction between two charged particles at rest | ||||||
| Who discovered it? | French physicist Charles-Augustin de Coulomb | ||||||
| What does it calculate? | The amount of force between two electrically charged particles at rest | ||||||
| What is the formula? | {\displaystyle | F | =k_{\text}{\frac { | q_{1} | q_{2} | }{r^{2}}}} | |
| What are the variables? | ke is a constant, q1 and q2 are the quantities of each charge, and the scalar r is the distance between the charges | ||||||
| What is the direction of the force? | Along the straight line joining the two charges | ||||||
| What is the nature of the force? | If the charges have the same sign, they repel each other; if they have different signs, they attract each other | ||||||
| What is the force called? | Electrostatic force or Coulomb force | ||||||
| What is Coulomb's constant? | In SI units, the constant k has the value 8.987 x 109 Nm2/C^2 | ||||||
| What are the similarities between Coulomb's Law and Newton's Law of Universal Gravitation? | Both are inverse-square laws; analogous roles of mass and charge | ||||||
| What are the differences between the two laws? | Gravitational constant G is much smaller than k; only one type of mass exists, whereas two types of electric charge exist |
What You'll Learn

The electrostatic force between two charged particles
Coulomb's Law is a mathematical formula that describes the electrostatic force between two charged particles. It was first proposed by French physicist Charles Coulomb in 1785 and is considered the starting point of the theory of electromagnetism.
> |F| = ke * (|q1| * |q2|) / r^2
Where:
- |F| is the magnitude of the attractive or repulsive electrostatic force between the two particles
- Ke is a constant, also known as Coulomb's constant, approximately equal to 8.9875 10^9 Nm^2/C^2
- |q1| and |q2| are the magnitudes of the charges of the two particles
- R is the distance between the charges
The force acts along the line joining the two charges. If the charges have the same sign, the electrostatic force between them is repulsive; if they have different signs, the force is attractive.
Coulomb's Law is an inverse-square law, meaning that the force decreases as the square of the distance between the two particles increases. This is similar to Isaac Newton's inverse-square law of universal gravitation, but gravitational forces are always attractive, whereas electrostatic forces can be attractive or repulsive.
- The charges must be stationary, i.e. they cannot move with respect to each other.
- The charges must be point charges or have a spherically symmetric distribution, such as a charged metal sphere. Charges with other distributions will not follow an inverse-square law.
- The charges must not overlap; they must be distinct and have a minimal distance between them.
By inputting the magnitudes of the charges and the distance between them into the formula, the electrostatic force between the two charged particles can be determined.
Community Property Laws: Tribal Land Exemption
You may want to see also

The inverse-square nature of Coulomb's Law
Coulomb's Law, or Coulomb's inverse-square law, is a fundamental principle in physics that describes the electrostatic force between two charged particles. The law was formulated by French physicist Charles-Augustin de Coulomb and published in 1785. It states that the magnitude of the force between two charged particles is directly proportional to the product of their charges and inversely proportional to the square of the distance between them. This relationship can be expressed mathematically as:
> F ∝ q1q2/r^2
Where:
- F is the electrostatic force between the charges
- Q1 and q2 are the magnitudes of the two charges
- R is the distance between the charges
The constant of proportionality in Coulomb's Law, k, is given by:
> k = 1 / (4πϵ0)
Where:
Ε0 is the electric constant or permittivity of free space, approximately equal to 8.854 x 10^-12 C^2·N^-1·m^-2
The inverse-square relationship in Coulomb's Law means that the force between two charged particles decreases rapidly as the distance between them increases. This has important implications for the behaviour of charged particles and the stability of ionic compounds. For example, in an ionic crystal lattice, the electrostatic attraction between oppositely charged ions decreases as the distance between them increases, leading to a more stable compound. Conversely, the electrostatic repulsion between like charges decreases as the distance between them increases, reducing the stability of the compound.
Coulomb's Law also holds within atoms, accurately describing the force between the positively charged atomic nucleus and the negatively charged electrons. It also correctly accounts for the forces that bind atoms and molecules together in solids and liquids. As the distance between ions increases, the force of attraction and binding energy approach zero, making ionic bonding less favourable. Conversely, as the magnitude of opposing charges increases, the energy increases, making ionic bonding more favourable.
The Levitical Priesthood: Laws and Regulations Explained
You may want to see also

The role of mass and charge
Coulomb's law is a mathematical formula that describes the electrostatic interaction between two charged particles. It was first proposed by French physicist Charles-Augustin de Coulomb in 1785. The law states that the magnitude of the attractive or repulsive electrostatic force between two charged particles is directly proportional to the product of their charges and inversely proportional to the square of the distance between them.
The force described by Coulomb's law acts along the line joining the centres of the two charged objects. Like charges repel each other, while unlike charges attract. The size of the force is directly proportional to the value of each charge. If the charges have the same sign, the force is repulsive, while if they have different signs, the force is attractive.
Coulomb's law can be applied to any number of point charges. The force acting on a small charge due to a system of charges is the vector addition of the individual forces acting alone on that charge due to each one of the charges. The resulting force vector is parallel to the electric field vector at that point, with that charge removed.
Nobility and the Law: Who Was Exempt?
You may want to see also

The force between charged objects
Coulomb's Law is a mathematical description of the electric force between charged objects formulated by 18th-century French physicist Charles-Augustin de Coulomb. It is analogous to Isaac Newton's law of gravity, with both laws following an inverse square law. However, gravitational forces always result in attraction, while electrostatic forces can result in either attraction or repulsion.
The force between two point charges is described by Coulomb's Law, which states that the force is directly proportional to the product of the charges and inversely proportional to the square of the distance between them. This can be expressed by the following equation:
$$F = \frac{1}{4 \pi \varepsilon_0} \frac{q1 q2}{r^2}$$
Where ε0 is the electric permittivity constant of free space, and q1 and q2 are the magnitudes of the two charges. The direction of the force is determined by the charges' polarity, with like charges repelling each other and opposite charges attracting.
The magnitude and direction of the electrostatic force are influenced by several variables, including the magnitude of the charges, the distance between them, and the medium between them. The force decreases rapidly as the distance between the charges increases and can be affected by the presence of different materials, such as air, vacuum, or insulating materials.
Coulomb's Law is essential for understanding the behaviour of charged particles and has been extensively tested and verified through experiments. It also serves as a foundation for the development of electromagnetism and related theories.
Artisan Labor Laws: Do NGOs Have Exemptions?
You may want to see also

The distance between charged objects
Coulomb's Law is a mathematical formula that describes the electrostatic interaction between two charged particles. The law states that the magnitude of the attractive or repulsive electrostatic force between two charged particles is directly proportional to the product of their charges and inversely proportional to the square of the distance between them.
The electrostatic force between two charged objects can be positive or negative, depending on whether the objects are attracted to or repelled by each other. Positive and negative charges are attracted to each other, whereas similar charges (two positive or two negative) repel each other. As the distance between charged objects increases, the force and electric fields decrease.
Coulomb's Law can be used to calculate the distance between charged objects. For example, an engineer measures the force between two ink drops and finds that each member of the pair exerts a repulsive force of 0.0000025 N on its partner. Using Coulomb's Law, the distance between the ink drops is calculated to be 130 microns.
Coulomb's Law is applicable to charged objects that are not moving relative to each other. It is easiest to apply to spherical objects or objects that are much smaller than the distance between them, in which case the objects can be approximated as spheres.
Newton's Laws and Rocket Nose Design
You may want to see also
Frequently asked questions
Coulomb's Law is a mathematical formula that describes the electrostatic force between two charged particles at rest. It was first proposed by French physicist Charles-Augustin de Coulomb in 1785. The law states that the force between two charges is directly proportional to the product of their magnitudes and inversely proportional to the square of the distance between them.
Coulomb's Law can be used to calculate the force between two ions. The formula for the electrostatic force is:
> |F| = ke * (|q1| * |q2|) / r^2
Where:
- ke is Coulomb's constant (approximately 9 x 10^9 Nm^2/C^2)
- q1 and q2 are the magnitudes of the charges
- r is the distance between the charges
By inputting the values for the charges and distance into this equation, you can calculate the force between the ions.
Coulomb's Law has several limitations. It only applies to charged objects that are stationary with respect to each other. Additionally, it is most easily applied to spherical objects or objects much smaller than the distance between them. Finally, it is an inverse-square law, meaning the force depends on the square of the denominator.
To determine the charge of an ion using Coulomb's Law, you need to know the force between the ion and another charged object, as well as the distance between them. You can then rearrange the equation to solve for the charge. Here is an example:
Let's say you have an unknown charge, q, and you measure a force of F between this charge and another charge of -2C when they are r meters apart. You can use Coulomb's Law to calculate q:
F = ke * (|q| * |-2|) / r^2
Solving for q: q = F * r^2 / (ke * |-2|)