Gases are highly relevant to our daily lives, from the air we breathe to the weather we experience. Gas laws are scientific principles that explain how properties like volume and pressure influence the behaviour of gases. The ideal gas law, for instance, describes the relationship between pressure, volume, temperature, and the number of gas molecules. This law is applied in various everyday scenarios, such as the inflation of airbags during a car crash or the compression of gases in propane tanks. Boyle's Law, Charles's Law, and Avogadro's Law are additional gas laws that govern the behaviour of gases and have practical applications in fields like medicine and meteorology.
| Characteristics | Values |
|---|---|
| Relationship | Pressure, temperature, volume and amount of gas present |
| Boyle's Law | Pressure of a gas is inversely proportional to volume of a gas |
| Avogadro's Law | Volume of a gas is proportional to the number of moles of a gas |
| Charles' Law | Volume of a gas is proportional to the temperature of a gas |
| Ideal Gas Law | Pressure and volume of a gas are proportional to each other |
What You'll Learn
- Boyle's Law: Pressure and volume are inversely proportional
- Avogadro's Law: Volume is proportional to the number of moles
- Charles' Law: Volume is proportional to temperature
- Dalton's Law: Total pressure of a gas mixture is the sum of its gases
- Ideal Gas Law: Pressure and volume are directly proportional to the number of moles and temperature

Boyle's Law: Pressure and volume are inversely proportional
Gas laws are a set of rules that explain how properties such as volume and pressure influence the behaviour of gases. One of the most well-known gas laws is Boyle's Law, which states that the pressure exerted by a gas is inversely proportional to the volume it occupies, as long as the temperature and quantity of the gas remain constant.
Boyle's Law can be mathematically expressed as P1V1 = P2V2, where P represents the pressure exerted by the gas and V represents the volume occupied by it. This relationship can also be written as PV = K, where K is a constant. This equation demonstrates that as the volume of a gas increases, its pressure decreases, and vice versa, as long as the temperature and quantity remain the same.
The discovery of this law is credited to Robert Boyle, an Anglo-Irish chemist who conducted experiments in 1662 to study the relationship between the pressure and volume of a confined gas held at a constant temperature. Boyle found that manipulating the pressure caused a corresponding change in volume, but in the opposite direction. This led to the formulation of Boyle's Law, which has since been supported by graphical analysis of Boyle's data.
The implications of Boyle's Law can be observed in various real-life scenarios. For example, when you blow air into a balloon, the pressure of the air causes the balloon to expand. If one end of the balloon is squeezed, reducing its volume, the pressure inside increases, causing the un-squeezed portion to expand outward. Similarly, when you inhale, your diaphragm increases the volume of your lungs, leading to a decrease in lung pressure and allowing atmospheric pressure to fill your lungs with air. The reverse occurs when you exhale.
Drone Journalism: Legal Boundaries and Ethical Concerns
You may want to see also

Avogadro's Law: Volume is proportional to the number of moles
Gas laws are a set of rules that explain how properties such as volume and pressure affect the behaviour of gases. Avogadro's Law, also known as Avogadro's hypothesis or principle, is one such law. It states that the volume of a gas is proportional to the number of moles of the gas. In other words, if you increase the volume of a gas, the number of moles of the gas will also increase, and vice versa.
Mathematically, Avogadro's Law can be written as:
V ∝ n
Where V is the volume of the gas and n is the amount of substance (in moles). This can also be written as:
V = k * n
Or
V/n = k
Where k is a constant for a given temperature and pressure. This means that for a given mass of an ideal gas, the volume and the number of moles are directly proportional if the temperature and pressure remain constant.
Avogadro's Law can be applied to compare the same substance under different conditions. For example, if you have two states of the same gas with different volumes (V1 and V2) and different moles (n1 and n2), you can use the equation:
V1/n1 = V2/n2
This equation shows that the ratio of volume to the number of moles remains constant for a given gas at a constant temperature and pressure.
Avogadro's Law has practical applications in various fields. For instance, it can be used to calculate the quantity of gas in a container or to determine the molar volume of an ideal gas under standard temperature and pressure (STP) conditions.
Transit Vehicles and Seat Belt Laws: Who's Exempt?
You may want to see also

Charles' Law: Volume is proportional to temperature
Charles's Law states that the volume of a gas is proportional to the temperature of a gas. In other words, if you heat a fixed amount of gas at constant pressure, the volume of the gas will increase proportionally to the increase in temperature. This law can be demonstrated by observing what happens to an inflated football that has been kept indoors when you take it outside on a cold day—the ball will get smaller.
Propane distributors take advantage of Charles's Law by lowering the temperature of propane to -42.2° Celsius (-44 Fahrenheit). This converts propane to a liquid, which is easier to transport and store. As the temperature drops, the gas molecules get closer together and the volume decreases.
Charles's Law can also be applied to everyday situations, such as breathing. People who ascend to high altitudes experience Charles's Law when they try to breathe. As they climb higher, the partial pressure of oxygen decreases as the total atmospheric pressure decreases. This can lead to Hypoxia, a serious medical condition that can potentially result in death.
Charles's Law can be used to calculate the temperature, pressure, and volume of any gas. It is one of the gas laws, which also include Boyle's Law and Avogadro's Law. These laws help us understand the relationship between pressure, temperature, volume, and the amount of gas present.
Congress and Slzndsr Laws: Who's Exempt?
You may want to see also

Dalton's Law: Total pressure of a gas mixture is the sum of its gases
Gas laws are scientific principles that explain how properties such as volume and pressure affect the way gases behave. One of these laws is Dalton's Law, also known as the Law of Partial Pressures.
Dalton's Law states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of the individual gases in the mixture. This empirical law was first observed by John Dalton in 1801 and published in 1802.
Mathematically, the pressure of a mixture of non-reactive gases can be defined as:
\co: 4>p_{total} = p_1 + p_2 + p_3 + ... + p_n\>
Where p1, p2, p3, etc. represent the partial pressures of each component gas.
The partial pressure of a gas is a measure of the thermodynamic activity of its molecules. Gases dissolve, diffuse, and react according to their partial pressures, rather than their concentrations in gas mixtures or liquids. This property of gases is also true in chemical reactions of gases in biological systems. For example, the necessary amount of oxygen for human respiration is determined by the partial pressure of oxygen alone, regardless of the concentration of oxygen in the inhaled breathing gases or dissolved in the blood.
Dalton's Law is based on the kinetic theory of gases, which states that a gas will diffuse to fill the container it is in and that there are no forces of attraction between the gas molecules. Therefore, the molecules in a mixture of gases are so far apart that they act independently and do not react with each other. Each gas exerts its own pressure on the system, and these pressures can be added together to find the total pressure of the gas mixture.
It's important to note that Dalton's Law does not strictly apply to real gases, and the deviation from the law increases with pressure. This is because, under high-pressure conditions, the volume occupied by the molecules becomes significant compared to the free space between them, and intermolecular forces become substantial enough to change the pressure exerted by the gas molecules.
International Law in Palestine: Applicable or Not?
You may want to see also

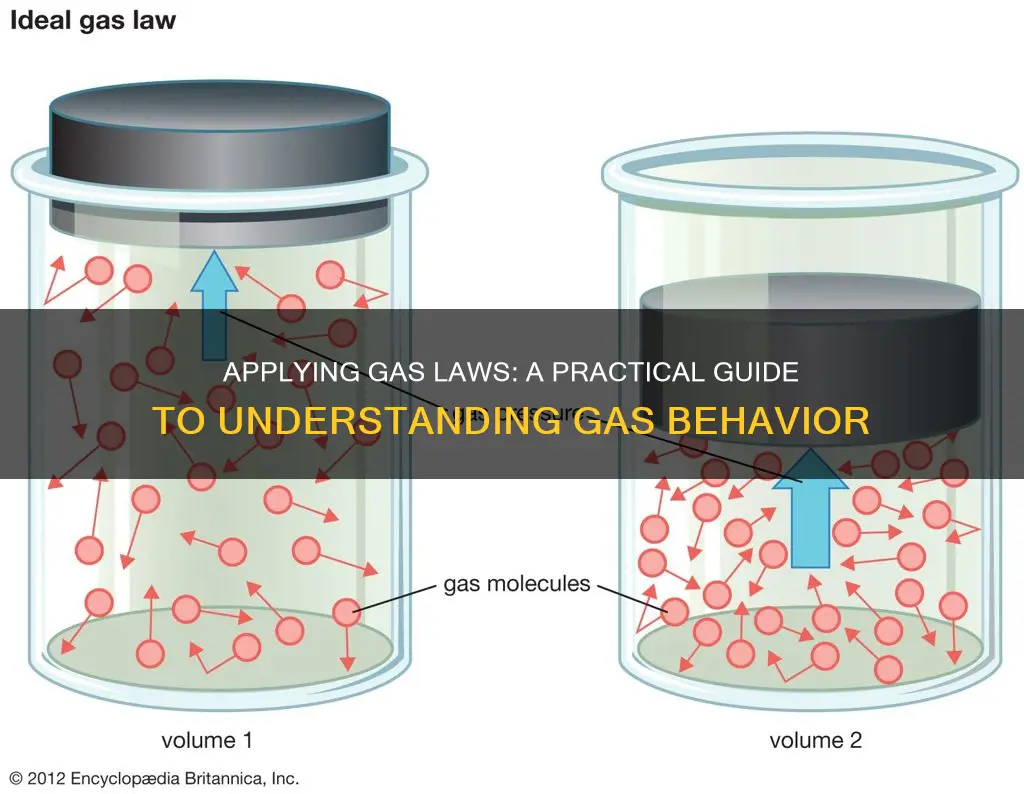
Ideal Gas Law: Pressure and volume are directly proportional to the number of moles and temperature
The Ideal Gas Law combines the Simple Gas Laws (Boyle's Law, Charles' Law, and Avogadro's Law) into a single equation. The Ideal Gas Law is:
PV = nRT
Where:
- P is the pressure of a gas
- V is its volume
- N is the number of moles of the gas
- T is its temperature on the Kelvin scale
- R is the gas constant
The Ideal Gas Law states that pressure and volume are directly proportional to the number of moles and temperature. This means that if you increase the number of moles of a gas and keep the temperature constant, the volume and pressure will increase. Similarly, if you increase the temperature of a gas and keep the number of moles constant, the volume and pressure will increase.
For example, let's consider a gas with a pressure of 1 atm, a volume of 2 L, a temperature of 273 K, and 1 mole. If we increase the number of moles to 2 while keeping the temperature constant, the new pressure and volume can be calculated using the Ideal Gas Law:
P1V1 = n1R1T1
P2V2 = n2R2T2
Given that R and T are constant, we can rearrange the equation to solve for V2:
V2 = n2 * (P2 * R * T) / (n * P)
V2 = 2 * (1 atm * R * 273 K) / (1 * 1 atm)
V2 = 2 L
As you can see, when we doubled the number of moles, the volume also doubled. The same principle applies to temperature. If we increase the temperature from 273 K to 546 K while keeping the number of moles constant, the new pressure and volume can be calculated as follows:
V2 = n * (P2 * R * T2) / (P * T1)
V2 = 1 * (1 atm * R * 546 K) / (1 atm * 273 K)
V2 = 2 L
Again, we see that when we doubled the temperature, the volume also doubled. This illustrates the direct proportionality between pressure and volume with the number of moles and temperature stated by the Ideal Gas Law.
Labor Laws: Do Your Children Count as Employees?
You may want to see also
Frequently asked questions
Gas laws outline the relationships between the pressure, volume, temperature, and amount of gas present. These factors influence how gases behave.
Boyle's Law states that the pressure of a gas is inversely proportional to its volume. For example, when a scuba diver exhales, the bubbles grow larger as they rise to the surface due to reduced water pressure.
Avogadro's Law states that the volume of a gas is directly proportional to the number of moles of the gas, given that temperature and pressure remain constant. An example of this is the breathing process, where the volume of the lungs increases when inhaling and decreases when exhaling.







