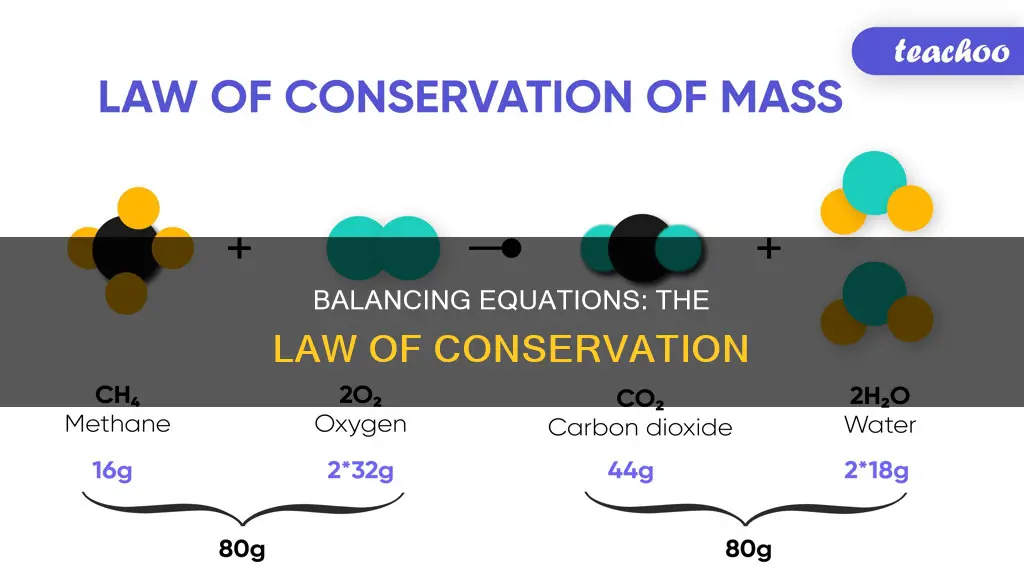
Balancing equations is a fundamental concept in chemistry that demonstrates the law of conservation of mass. This principle, also known as the conservation of matter, was discovered by Antoine Lavoisier in 1789 and states that mass remains constant in a chemical reaction. In other words, matter cannot be created or destroyed, only rearranged. When balancing equations, the goal is to ensure that the number of atoms on each side of the equation is equal, providing a precise representation of the chemical reaction and allowing for accurate predictions of reactant and product quantities.
| Characteristics | Values |
|---|---|
| Name of Law | Law of Conservation of Mass |
| Description | States that matter cannot be created or destroyed |
| Equation Balancing | Only change the coefficients (the numbers in front of substances) |
| Never change the subscripts (the small numbers after elements) |
What You'll Learn

Conservation of mass
Balancing chemical equations involves ensuring that the number of atoms of each element is the same on both sides of the equation. This process is guided by the law of conservation of mass, which states that matter cannot be created or destroyed. In other words, the total weight of a reaction cannot change, and the mass of the reactants and products must be the same.
The law of conservation of mass, also known as the conservation of matter, is a fundamental principle in science. It was first discovered by Antoine Lavoisier in 1789, although the Greek philosopher Anaxagoras had previously stated a similar idea in the fifth century. Lavoisier's work demonstrated that the mass of the reactants in a chemical reaction must equal the mass of the products, as matter cannot be created or destroyed.
Balancing equations involves manipulating the coefficients, or numbers in front of molecules or atoms, to ensure that the number of atoms of each element is equal on both sides. The subscripts, or smaller numbers after atoms, cannot be changed. For example, in the equation N2 + H2 -> NH3, the balanced equation would be N2 + 3H2 -> 2NH3, where the coefficients of The law being applied when balancing chemical equations is the Law of Conservation of Mass. This law is an important guiding principle in science. It states that matter cannot be created or destroyed, and as such, the number of atoms on the left-hand side (LHS) of a chemical equation must always equal the number of atoms on the right-hand side (RHS). In other words, the total mass of the reactants must be equal to the total mass of the products. This is because the mass stays constant during a chemical reaction.
The Law of Conservation of Mass was born out of Antoine Lavoisier's discovery in 1789 that matter cannot be created or destroyed. However, he was not the first person to notice this principle. During the fifth century, the Greek philosopher Anaxagoras posited that nothing can be created or destroyed because everything is a rearrangement of prior ingredients.
Balancing chemical equations involves ensuring that the number of atoms for each element is the same on both sides of the equation. This is achieved by changing the coefficients, or the numbers in front of the substances, rather than the subscripts, which are the smaller numbers after the elements. For example, in the equation N2 + H2 -> NH3, it would be balanced as N2 + 3H2 -> 2NH3, so that all the atoms match up on both sides.
The Law of Conservation of Mass is demonstrated in balancing equations because it shows that the mass in a chemical reaction remains constant. The total weight of a reaction cannot change, and so the mass of the reactants and products must be the same. This is why it is important to balance chemical equations accurately, as it ensures that the law is obeyed.
Title III Rules: Do They Govern Family Law?
You may want to see also

Mass cannot be created or destroyed
Balancing chemical equations involves ensuring that the number of atoms on each side of the equation is equal. This process is guided by the Law of Conservation of Mass, which states that mass cannot be created or destroyed. In other words, the total weight of a reaction cannot change, and the mass of the reactants and products must be the same. This law was discovered by Antoine Lavoisier in 1789, although the Greek philosopher Anaxagoras had previously asserted a similar principle in the 5th century.
The Law of Conservation of Mass is a fundamental principle in science. It demonstrates that the mass of a system remains constant, even during a chemical reaction. This is because the type of atoms does not change in a chemical reaction, and the number of atoms stays the same. As a result, the total mass of the reactants will match the total mass of the products. For example, in the reaction:
> H-H + H-H + O=O -> H-O-H + H-O-H
There are 4 hydrogen atoms and 2 oxygen atoms before the reaction, and the same number of each atom after the reaction. The total mass before the reaction is 36 amu (4x1 for hydrogen + 2x16 for oxygen), and the total mass after the reaction is also 36 amu.
To balance a chemical equation, you can only change the coefficients, or the numbers in front of the molecules or atoms. For example, in the equation N2 + H2 -> NH3, you would change it to N2 + 3H2 -> 2NH3 so that all the atoms match up on both sides.
Meiosis and Mendel's Law: Segregating Chromosomes
You may want to see also

Ratio of reactants to products
Balancing chemical equations involves ensuring that the ratio of reactants to products results in the total number of atoms of reactants matching the number of atoms of the products. This is because the type of atoms in a chemical reaction does not change, and neither does the number of atoms—only their arrangement.
For example, consider the chemical equation:
> H-H + H-H + O=O -> H-O-H + H-O-H
There are 4 hydrogen (H) atoms and 2 oxygen (O) atoms on both sides of the equation. The total mass before and after the reaction is also the same: 4x1 (for hydrogen) + 2x16 (for oxygen) = 36amu.
This balance of atoms and mass is in accordance with the Law of Conservation of Mass, which states that matter cannot be created or destroyed. In other words, the total weight of a reaction cannot change, and the mass before the reaction must be the same as the mass after the reaction. This law was first discovered by Antoine Lavoisier in 1789, although the Greek philosopher Anaxagoras had previously asserted the same principle as early as the fifth century.
Cell Phone Laws: Parking Lot Exempt?
You may want to see also

Coefficients are numbers in front of formulas
Balancing chemical equations involves ensuring that the number of each type of atom is the same on both sides of the equation. This process is guided by the Law of Conservation of Mass, which states that matter cannot be created or destroyed.
Coefficients are the numbers placed in front of the formulas in a chemical equation. They represent the relative number of molecules or atoms of each substance involved in the reaction. For example, in the balanced equation for the reaction between hydrogen gas and oxygen gas to form water:
> 2H2 + O2 → 2H2O
The coefficient "2" in front of the hydrogen gas (H2) means that there are two molecules of hydrogen gas for every molecule of oxygen gas (O2). The coefficient "2" in front of the water (H2O) means that two molecules of water are produced for every two molecules of hydrogen gas and one molecule of oxygen gas.
Coefficients are crucial to balancing chemical equations because they ensure that the same number of atoms of each element is present on both the reactant and product sides of the equation. By adjusting the coefficients, chemists can change the relative amounts of reactants and products in a chemical reaction.
In summary, coefficients represent the number of molecules or atoms involved in the reaction and the number of moles of each substance. They are essential for understanding and using chemical equations successfully.
US Law Abroad: How Far Does It Reach?
You may want to see also

Subscripts are small numbers after elements
Balancing chemical equations involves ensuring that the number of each type of atom is the same on both sides of the equation. This process is guided by the Law of Conservation of Mass, a fundamental principle in science. According to this law, it is impossible to create or destroy matter, and the total weight of a reaction must remain constant. In other words, the mass of the reactants and products must be equal.
Subscripts, which are the small numbers found after elements, play a crucial role in balancing chemical equations. Unlike coefficients (the numbers in front of substances), subscripts must never be changed when balancing an equation. For example, in the equation N2 + H2 -> NH3, you would adjust the coefficients to achieve balance, resulting in N2 + 3H2 -> 2NH3. The subscripts remain unchanged, ensuring that the chemical formulae of the molecules or compounds remain accurate.
The Law of Conservation of Mass, also known as the Conservation of Matter, dictates that the total number of atoms of reactants must match the number of atoms of products. This principle is based on the understanding that the type of atoms does not change in a chemical reaction (excluding nuclear processes), and the number of atoms remains constant. As a result, the total mass of the reactants will be equal to the total mass of the products.
Consider the reaction of hydrogen and oxygen to form water:
2H2 + O2 -> 2H2O
In this equation, the subscripts of 2 for hydrogen (H) and oxygen (O) remain fixed. The coefficient of 2 is introduced in front of H2O to balance the equation. This ensures that there are two oxygen atoms on each side of the equation, adhering to the Law of Conservation of Mass.
By preserving the subscripts, we maintain the integrity of the chemical formulae and the identities of the substances involved in the reaction. Changing the subscripts would alter the very nature of the molecules or compounds, which is not permitted when balancing equations.
Labor Laws: Do They Apply to Hospital Work?
You may want to see also
Frequently asked questions
The law being applied is the Law of Conservation of Mass.
The law states that matter cannot be created or destroyed.
Balancing equations ensures that the number of atoms on both sides of the equation is the same. This is important as it accurately represents what happens in the real world during a chemical reaction.
To balance equations, you must ensure that the number of atoms for all elements is the same on both sides. You can only change the coefficients (the numbers in front of the molecules or atoms) to achieve this.







