Refrigeration is a critical process in our daily lives, from preserving food and medicines to cooling machines and spaces. It works by removing heat from one area and transferring it to another, creating a cooling effect. This process is based on the principles of thermodynamics, specifically the transfer of heat and the manipulation of temperature and pressure. The second law of thermodynamics, which states that heat will always flow spontaneously from hotter to colder substances, is particularly relevant to the functioning of refrigeration systems. This law also asserts that it is impossible for heat to spontaneously flow from a colder body to a hotter one without external work being done, which is the fundamental concept behind refrigeration.
| Characteristics | Values |
|---|---|
| Law of Thermodynamics that applies to Refrigerants | Second Law of Thermodynamics |
| Description | Describes the limitations of heat transfer |
| Refrigeration Statement | "Heat will always flow spontaneously from hotter substances to colder ones" |
| Waste Heat Statement | "It is impossible to extract an amount of heat, represented as QH, from a hot reservoir and use it all to do work." |
| Clausius Statement | "The entropy of a closed system can never decrease." |
| Kelvin-Planck Statement | "It is impossible for the thermal efficiency of an engine to be 100%, where all of the heat from the hot body would be transferred to useful work." |
What You'll Learn

The Second Law of Thermodynamics
> "Heat can never pass from a colder to a warmer body without some other change, connected therewith, occurring at the same time."
The Second Law establishes the concept of entropy as a physical property of a thermodynamic system. Entropy is the degree of randomness or disorder in a system. The law predicts whether processes are forbidden despite obeying the requirement of conservation of energy as expressed in the First Law of Thermodynamics. It also provides necessary criteria for spontaneous processes. For example, the First Law allows for a cup to fall off a table and break, as well as for the reverse process of the fragments coming back together and jumping back onto the table, while the Second Law allows the former but denies the latter.
The Second Law can be formulated by the observation that the entropy of isolated systems left to spontaneous evolution cannot decrease, as they always tend toward a state of thermodynamic equilibrium where the entropy is highest at the given internal energy. This increase in the combined entropy of a system and its surroundings accounts for the irreversibility of natural processes, often referred to in the concept of the arrow of time.
The Second Law also states that not all heat can be converted into work in a cyclic process. This is known as the Kelvin statement, formulated by William Thomson, or Lord Kelvin:
> "It is impossible to convert heat completely in a cyclic process."
This means that there is no way to convert all the energy of a system into work without losing energy.
The Second Law can be expressed in many ways, but it always affirms that the entropy of the universe, as an isolated system, will always increase over time, and that the changes in entropy in the universe can never be negative.
Understanding Romeo and Juliet Law Exemptions in Ohio
You may want to see also

The Clausius Statement
> "Heat can never pass from a colder to a warmer body without some other change, connected therewith, occurring at the same time."
In simpler terms, the statement asserts that heat will always flow spontaneously from hotter substances to colder ones. This is a fundamental principle in understanding how refrigeration works. Refrigerators transfer heat from the cold regions inside the device to hot regions outside, thereby cooling the internal temperature. This process occurs because heat naturally flows from hotter to colder regions, as outlined in the Clausius Statement.
The statement also highlights that heat transfer can only occur spontaneously in the direction of temperature decrease. This has significant implications for refrigeration systems, as it means that external work is required to force heat to move from a colder region to a hotter one. This is why refrigerators require energy input to function and cannot operate without any work being done on them.
Understanding Snell's Law Applicability to Sound Waves
You may want to see also

The Kelvin-Planck Statement
> "It is impossible to construct a device that operates on a cycle and produces no other effect than the transfer of heat from a single body to produce work."
This statement is a special case of the second law of thermodynamics. It is also known as the heat engine statement. The statement highlights that work can be fully converted into heat, but heat cannot be fully converted into work. This is why work is called high-grade energy, and heat is called low-grade energy.
> "No process is possible whose sole result is the transfer of heat from a colder object to a warmer object."
Both statements refer to different cycles, but they describe the same physical impossibility. Any device that violates the Kelvin-Planck Statement must also violate the Clausius Statement, and vice versa. Together, they establish the universal nature of irreversible processes in thermodynamics.
Understanding California's Lemon Law: After Warranty Rights
You may want to see also

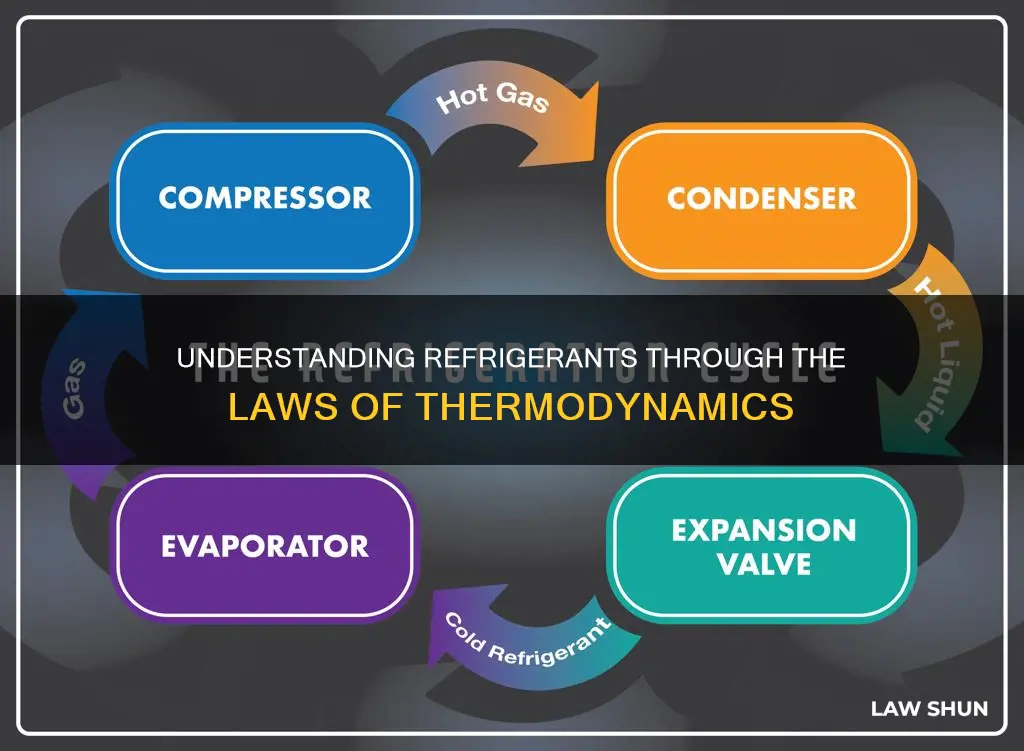
The Refrigeration Cycle
The expansion device is the next step in the cycle, where the high-pressure liquid refrigerant experiences a drop in pressure, causing some of it to boil and create a two-phase mixture. This rapid phase change is known as flashing. The refrigerant then enters the evaporator, which is the second heat exchanger in the circuit. The evaporator absorbs heat, cooling the air around it. A fan forces air across the evaporator's fins, and the refrigerant absorbs this heat and turns back into a gas.
After completing its journey through the evaporator, the refrigerant returns to the compressor as a gas, and the cycle starts over again. This continuous cycle helps to keep the inside of a refrigerator or air-conditioned space cool.
Tennessee's Hands-Free Law: Commercial Vehicles Included?
You may want to see also

The Continuous Loop
The refrigeration cycle is a continuous loop, with the refrigerant undergoing the same sequence of transformations repeatedly. This perpetual process ensures a sustained cooling effect within the refrigerated space. The cycle begins with the refrigerant being drawn into the compressor and released at a higher temperature. The compressor then pushes out the refrigerant in the condenser coils outside the refrigerator, where it exchanges its heat with the room-temperature air.
As the hot refrigerant in the condenser coils meets the cooler air, it becomes a liquid. This liquid refrigerant then passes through a metering device, which causes it to expand rapidly, resulting in a significant drop in temperature. This expanded liquid refrigerant then enters the evaporator, where it absorbs heat from the surrounding air, causing its temperature to rise and resulting in a phase change from liquid to vapour. The refrigerant then returns to the compressor, and the cycle repeats.
The fact that the system operates in a closed loop allows the refrigerant to be recycled through the system, eliminating the need for its continual recharging. This closed-loop system is essential to the continuous loop of the refrigeration cycle, ensuring the efficient and sustained cooling effect that keeps our food fresh and our homes comfortable.
Law Training Contracts: Application Strategies for Success
You may want to see also
Frequently asked questions
Refrigeration is the process of removing heat from one area and transferring it to another, resulting in a cooling effect in the desired space. This is achieved through a closed-loop system where a refrigerant absorbs and releases heat, changing from a liquid to a gas and back.
The Second Law states that heat will always flow spontaneously from hotter substances to colder ones. This is fundamental to refrigeration, as it explains why heat is transferred from the inside of a refrigerator to the outside, creating a cooling effect within.
The compressor is often referred to as the heart of the system. It circulates the refrigerant through the closed-loop system and compresses it, increasing its pressure and temperature. This compression facilitates the phase change of the refrigerant from a gas to a liquid.
The First Law, also known as the law of energy conservation, states that energy cannot be created or destroyed, only converted from one form to another. In the context of refrigeration, it means that the temperature of a system can be raised by adding heat (thermal energy) or by doing work on it.







