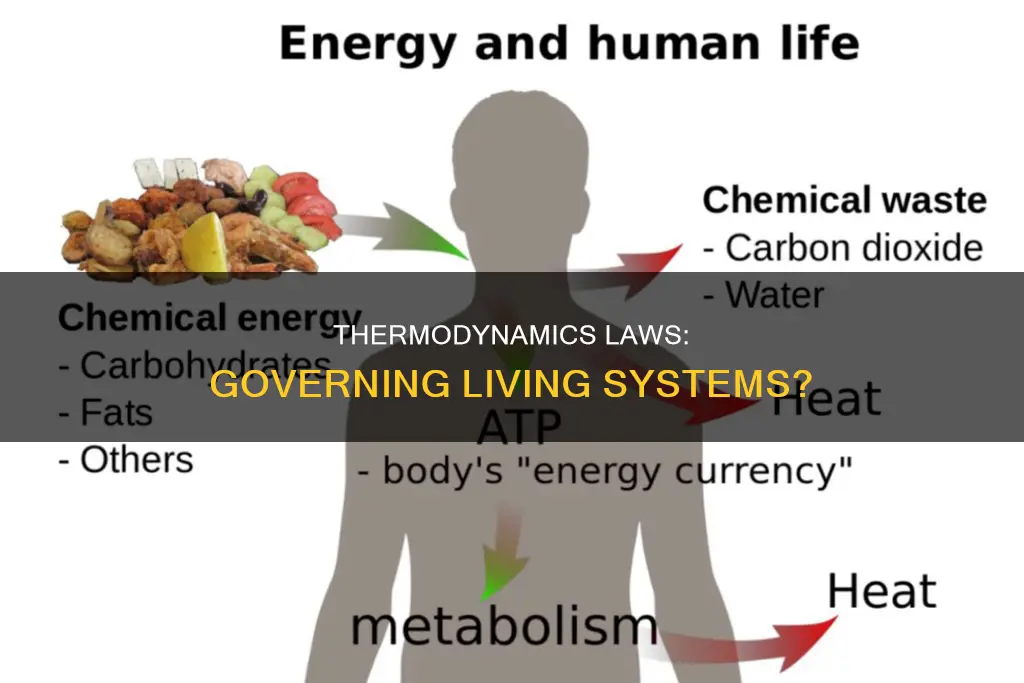
The laws of thermodynamics are fundamental principles that govern the behaviour of energy in the universe. These laws apply to all physical systems, including living organisms, which are open systems that constantly exchange energy with their surroundings. The first law of thermodynamics, also known as the law of conservation of energy, states that energy cannot be created or destroyed, only transformed from one form to another. This law is relevant to biological processes such as photosynthesis, where plants convert sunlight into chemical energy stored in glucose. The second law of thermodynamics states that when energy is transferred, there will always be a loss of energy, resulting in an increase in entropy or disorder in the system. Living organisms maintain a highly ordered state by constantly taking in energy, but they contribute to the overall increase in entropy in the universe by releasing energy and waste by-products into their surroundings.
What You'll Learn
- Living organisms are open systems
- The First Law of Thermodynamics states that energy can neither be created nor destroyed
- The Second Law of Thermodynamics states that energy transfers are never completely efficient
- Entropy is a measure of randomness or disorder in a system
- The Third Law of Biology states that all living organisms arose in an evolutionary process

Living organisms are open systems
The open nature of living systems is essential for their survival and functionality. They require a constant input of energy to maintain their highly ordered state and perform life processes. This energy is obtained from the sun through photosynthesis in plants and indirectly through the consumption of plants or other animals in the case of heterotrophs.
The cells that comprise living organisms are semi-permeable, allowing the passage of specific molecules and ions while blocking others. This selective permeability enables the acquisition of essential elements, nutrients, and genetic material while expelling waste products and toxins. The ability to take in novel genetic traits contributes to increased phenotypic variability and accelerated evolutionary divergence.
The open nature of living systems also means they are not in thermodynamic equilibrium with their surroundings. Living organisms actively maintain a state of dynamic stability, far from equilibrium, by continuously alternating cycles of energy consumption and release. This nonequilibrium state is a fundamental characteristic of life, allowing organisms to adapt and perform the work necessary for survival.
The concept of open systems is integral to understanding the thermodynamics of living organisms and their ability to sustain life processes, adapt to their environment, and evolve over time.
Understanding Minimum Wage Laws for Minors
You may want to see also

The First Law of Thermodynamics states that energy can neither be created nor destroyed
The First Law of Thermodynamics, also known as the law of conservation of energy, states that energy can neither be created nor destroyed. This means that the total amount of energy in the universe has always been and always will be the same. Energy can be moved from one place to another or transformed into a different form, but the total amount remains constant. For example, light bulbs transform electrical energy into light energy, and gas stoves convert natural gas chemical energy into heat energy.
The First Law of Thermodynamics applies to all systems in the universe, including living systems. Biological organisms are open systems, meaning they can exchange energy with their surroundings. They consume energy-storing molecules and release energy by doing work. For instance, plants convert the energy of sunlight into chemical energy stored within organic molecules through photosynthesis. This energy is then released through cellular respiration, allowing plant and animal organisms to access it and perform essential cell functions such as DNA replication and cell movement.
Living systems require a constant input of energy to maintain their highly ordered state. This energy is obtained from food or the sun and is then converted into other types of energy through a series of biochemical reactions. For example, in living cells, adenosine triphosphate (ATP), the primary source of energy for living organisms, is synthesized from food through a series of biochemical reactions.
The First Law of Thermodynamics highlights that energy is neither created nor destroyed but is continually transformed and transferred. This law applies to all systems, including living organisms, which obtain, transform, and utilize energy to sustain their highly ordered and complex structures and functions.
Domestic Violence Laws: Unmarried Couples' Rights Explored
You may want to see also

The Second Law of Thermodynamics states that energy transfers are never completely efficient
The laws of thermodynamics are indeed applicable to living systems, and they govern the chemical processes (metabolism) in all biological organisms. The First Law of Thermodynamics, also known as the Law of Conservation of Energy, states that energy can neither be created nor destroyed. It can, however, change from one form to another, and the energy in a closed system remains constant.
The Second Law of Thermodynamics, which states that energy transfers are never completely efficient, is of particular interest. When energy is transferred, there will always be less energy available at the end of the transfer process than at the beginning. This is due to the presence of entropy, which is a measure of disorder in a closed system. As energy is transferred, entropy increases, and not all of the available energy will be useful to the organism.
In biological systems, the transfer of energy is never 100% efficient. For example, during photosynthesis, plants do not absorb all of the light energy they receive from the sun. Some of it is reflected, while some is lost as heat. This loss of energy to the surrounding environment results in an increase in disorder, or entropy. Animals, unlike plants and other photosynthetic organisms, cannot generate energy directly from sunlight. They must consume plants or other animals for energy.
The higher an organism is on the food chain, the less available energy it receives from its food sources. A significant amount of energy is lost during the metabolic processes performed by the producers and primary consumers that are consumed. Consequently, there is much less energy available for organisms at higher trophic levels. This decrease in available energy limits the number of organisms that can be supported at these higher levels.
Living systems require a constant input of energy to maintain their highly ordered state. Cells, for example, are highly ordered and have low entropy. However, the processes performed to maintain this order result in an increase in entropy in the cell's surroundings. Thus, while individual cells may exhibit low entropy, the overall entropy of the universe increases due to the transfer of energy.
The concept of entropy is essential in understanding the Second Law of Thermodynamics and its application to living systems. It highlights the inherent inefficiencies in energy transfers, whether in biological or non-biological systems.
Understanding the Process: Applying for Lemon Law
You may want to see also

Entropy is a measure of randomness or disorder in a system
The laws of thermodynamics are indeed important unifying principles of biology, governing the chemical processes (metabolism) in all biological organisms. Living organisms are nonequilibrium thermodynamic systems, converting the energy of the sun and food into other types of energy.
The first law of thermodynamics, also known as the law of conservation of energy, states that energy can neither be created nor destroyed. In a closed system, the energy is transformed from one form to another, and the total amount of energy remains constant. This law applies to biological systems, where energy is required for survival and is transformed through various processes to maintain life.
The second law of thermodynamics is also relevant to living systems. It states that when energy is transferred, there will be less energy available at the end of the transfer process than at the beginning due to the presence of entropy. Entropy is a measure of randomness or disorder in a system. In the context of thermodynamics, it specifically refers to the measure of disorder in a closed system. As energy is transferred, entropy increases, and not all the available energy will be useful to the organism.
In biological systems, entropy is crucial in understanding the behaviour of cells. Cells are highly ordered systems with low entropy. However, to maintain this order, energy is lost to the surroundings or transformed, leading to an increase in entropy in the cell's surroundings. This increase in entropy is observed in various biological processes, such as photosynthesis and cellular respiration.
Furthermore, the concept of entropy is essential in understanding the relationship between living organisms and their surrounding environment. Living organisms maintain a state of low entropy or order within themselves, which is contrary to the tendency of their surroundings to increase in entropy or disorder. This difference in entropy between the organism and its environment allows for the creation of ordered biological structures.
Entropy also plays a role in the evolution of living organisms. Organisms must maintain their ordered structures for a sufficient period to allow for reproduction and the survival of their offspring. This time interval varies among species, with bacteria, for example, having a shorter interval due to their ability to divide cells quickly.
In summary, the laws of thermodynamics, including the concept of entropy, are applicable to living systems. Entropy, as a measure of randomness or disorder, helps explain the energy transformations and behaviours of biological organisms and their interactions with their environment.
Space Laws: Do Legal Boundaries Extend Beyond Earth?
You may want to see also

The Third Law of Biology states that all living organisms arose in an evolutionary process
The Third Law of Biology is supported by a substantial body of evidence. Firstly, it is widely accepted that all life forms share common ancestors. This is evident through the presence of homologous macromolecules, such as DNA, RNA, and proteins, which are derived from a common ancestor. Additionally, the universal genetic code serves as further proof of a shared evolutionary history.
Furthermore, Darwin's studies of natural selection provide strong support for the Third Law of Biology. Natural selection acts as the primary driver of genetic change, promoting evolutionary divergence and diversity. This mechanism explains how creatures as distinct as animals and plants could share a common ancestor.
The Third Law of Biology also accounts for the existence of functional genetic instructions (FGIs) in living organisms. FGIs are essential for life as they maintain stable, ordered states within cells, allowing them to perform specific metabolic functions. These FGIs are subject to genetic variation, which arises through gene transfer in prokaryotes and sexual reproduction in higher organisms. This genetic variation leads to increased phenotypic variability and accelerated evolutionary divergence within a population.
Moreover, the Third Law of Biology is consistent with our understanding of entropy and its relationship to evolution. Living organisms maintain order and low entropy within their cellular structures, despite existing in environments that tend towards increasing disorder or high entropy. This ability to create ordered structures by increasing local entropy is a fundamental aspect of evolution.
In conclusion, the Third Law of Biology is a fundamental principle that explains the evolutionary origins of all life on Earth. It accurately predicts the relatedness of organisms and accounts for their similarities and differences. This law is supported by evidence from genetics, evolutionary biology, and entropy, solidifying its importance in our understanding of the natural world.
Minimum Wage Laws: Contractors and Compliance
You may want to see also
Frequently asked questions
There are two laws of thermodynamics: the first law states that the total amount of energy in the universe is constant and cannot be created or destroyed, only transferred or transformed. The second law states that every energy transfer involves some loss of energy, resulting in a more disordered system.
Yes, living systems follow the first law. All biological organisms require a constant input of energy to survive, which is then transformed from one form to another. For example, plants convert light energy from the sun into chemical energy stored in the form of glucose.
Living systems do follow the second law. As they take in energy-storing molecules and transform them through chemical reactions, some energy is lost in the process as heat energy. This loss of energy results in an increase in entropy, or disorder, in the universe.
Living systems are not closed systems, meaning they can gain or lose energy from their external environment. This makes them open systems, which are not subject to the second law in the same way. Living organisms contain information in their DNA that allows them to obtain energy from outside their system and maintain their ordered state.
The laws of thermodynamics are important unifying principles of biology, governing the chemical processes (metabolism) in all biological organisms. They help explain how energy is transferred and transformed in living systems, and how these systems maintain their highly ordered state despite the increase in entropy in the universe.