The law of multiple proportions, also known as Dalton's Law, states that when two elements combine to form multiple compounds, the ratio of the weight of one element to a fixed weight of the other will be a ratio of small whole numbers. This law was formulated by John Dalton in 1803 and published in his 1804 work A New System of Chemical Philosophy. The law of multiple proportions is one of the basic rules in stoichiometry, a subdivision of chemistry. While the law is applicable to simple compounds, it often does not apply to very large molecules or non-stoichiometric compounds.
| Characteristics | Values |
|---|---|
| Definition | When two elements combine to form two or more compounds, the ratio of the masses of one element that combines with a fixed mass of the other are in a ratio of small whole numbers |
| Other Names | Law of simple multiple proportions, Dalton's Law |
| Discoverer | John Dalton |
| Year of Discovery | 1803 or 1804 |
| Examples | Nitrogen combines with oxygen to form five types of oxides; hydrogen combines with oxygen to form water and hydrogen peroxide |
| Applicability | Simple compounds, not large molecules or non-stoichiometric compounds |
| Importance | Helps understand the constituents that make up a compound, forms the basis of stoichiometry |
What You'll Learn

The law of multiple proportions applies to simple compounds
The law of multiple proportions, also known as Dalton's Law, was formulated by John Dalton in 1803 or 1804. The law states that when two elements combine to form two or more compounds, the ratio of the masses of one element that combines with a fixed mass of the other are in a ratio of small whole numbers.
Another example is the combination of nitrogen and oxygen to form five types of oxides: NO, NO2, NO3, NO4, and NO5. The weight of nitrogen remains the same at 14g across all these oxides, while the weight of oxygen increases in the order given, giving a ratio of 1:2:3:4:5.
The law of multiple proportions is one of the basic rules in stoichiometry, a subdivision of chemistry. It is based on the observation that when elements combine to form compounds, they do so in definite proportions. This led Dalton to develop the modern theory of atoms, as it suggested that elements combine in multiples of a basic quantity.
While the law of multiple proportions is applicable to simple compounds, it often does not apply to very large molecules or complex compounds. For example, when comparing the hydrocarbons decane (C10H22) and undecane (C11H24), the ratio of hydrogen masses is 121:120, which is not a ratio of small whole numbers.
Iowa Lemon Law: Does It Cover Campers and RVs?
You may want to see also

The law is also known as Dalton's Law
The law of multiple proportions, also known as Dalton's Law, was announced in 1803 by English chemist John Dalton. The law states that when two elements combine to form more than one compound, the weights of one element that combine with a fixed weight of the other are in a ratio of small whole numbers.
For example, there are five distinct oxides of nitrogen, and the weights of oxygen in combination with 14 grams of nitrogen are, in increasing order, 8, 16, 24, 32, and 40 grams, or in a ratio of 1:2:3:4:5.
The discovery of this pattern led Dalton to develop the modern theory of atoms, as it suggested that elements combine with each other in multiples of a basic quantity. This theory, known as Dalton's atomic theory, gained widespread interest shortly after he published it. However, it was not universally accepted as the law of multiple proportions by itself was not complete proof of the existence of atoms. It was not until other discoveries in chemistry and physics throughout the 19th century that atomic theory gained universal acceptance.
The law of multiple proportions forms the basis of stoichiometry, along with the law of definite proportions. However, it is important to note that this law often does not apply when comparing very large molecules. For example, when examining the hydrocarbons decane (C10H22) and undecane (C11H24), the ratio of hydrogen masses is 121:120, which deviates from the expected ratio of small whole numbers.
Homeland Law: Immigration Inclusions and Exemptions
You may want to see also

It does not apply to non-stoichiometric compounds
The law of multiple proportions states that when two elements combine to form multiple compounds, the ratio of the weights of one element that combines with a fixed weight of the other element will be small whole numbers. This law, also known as Dalton's Law, was formulated by John Dalton in 1803.
However, this law does not apply to non-stoichiometric compounds. Non-stoichiometric compounds are almost always solid inorganic compounds with elemental compositions that cannot be represented by a ratio of small whole numbers. In other words, the proportions of elements in these compounds cannot be expressed as simple whole-number ratios. Instead, their formulas often include variables or irrational numbers to account for the non-stoichiometric ratios.
The existence of non-stoichiometric compounds is due to defects in the lattice structures of crystalline substances. For example, in a sodium chloride crystal, if a sodium ion site is occupied by a neutral sodium atom, the compound becomes non-stoichiometric because it now has more sodium ions than chloride ions. This deviation from stoichiometry can also occur when some atoms are missing or when too many atoms are packed into the lattice structure.
Non-stoichiometric compounds are commonly found among transition metal oxides, sulfides, fluorides, hydrides, carbides, nitrides, and tellurides. They often exhibit special electrical or chemical properties due to their defects, such as enhanced electrical conductivity or unique magnetic properties. For instance, the mobility of hydrogen atoms within palladium hydride, a non-stoichiometric material, allows it to conduct hydrogen.
While the law of multiple proportions provides a useful framework for understanding the ratios of elements in many compounds, it does not encompass the complexities of non-stoichiometric compounds, which require a different approach to describe their elemental compositions accurately.
Castle Law: Exemptions for Young Adults?
You may want to see also

The law is a basic rule in stoichiometry
Stoichiometry is the study of the quantitative relationships between reactants and products in a chemical reaction. It is founded on the law of conservation of mass, which states that the total mass of the reactants is equal to the total mass of the products. This leads to the insight that the relations among the quantities of reactants and products typically form a ratio of positive integers.
The law of multiple proportions, also known as Dalton's Law, is a basic rule in stoichiometry. It states that when two elements combine to form multiple compounds, the ratio of the masses of one element that combines with a fixed mass of the other element are in a ratio of small whole numbers. For example, in methane (CH4) and ethane (C2H6), the ratio of hydrogen to carbon is 4:3. This discovery led John Dalton to develop the modern theory of atoms, as it suggested that elements combine in multiples of a basic quantity.
The law of multiple proportions is especially useful in stoichiometry because it allows us to determine the quantities of products and reactants in a chemical reaction. By knowing the amounts of the separate reactants, we can calculate the amount of the product. Conversely, if we know the quantity of one reactant and can empirically determine the quantity of the products, we can calculate the amount of the other reactants.
It is important to note that the law of multiple proportions may not apply when comparing very large molecules. In such cases, the ratios of the elements may not be in small whole numbers.
HIPAA Laws: Do They Apply to the President?
You may want to see also

The discovery of this law led to the development of the modern theory of atoms
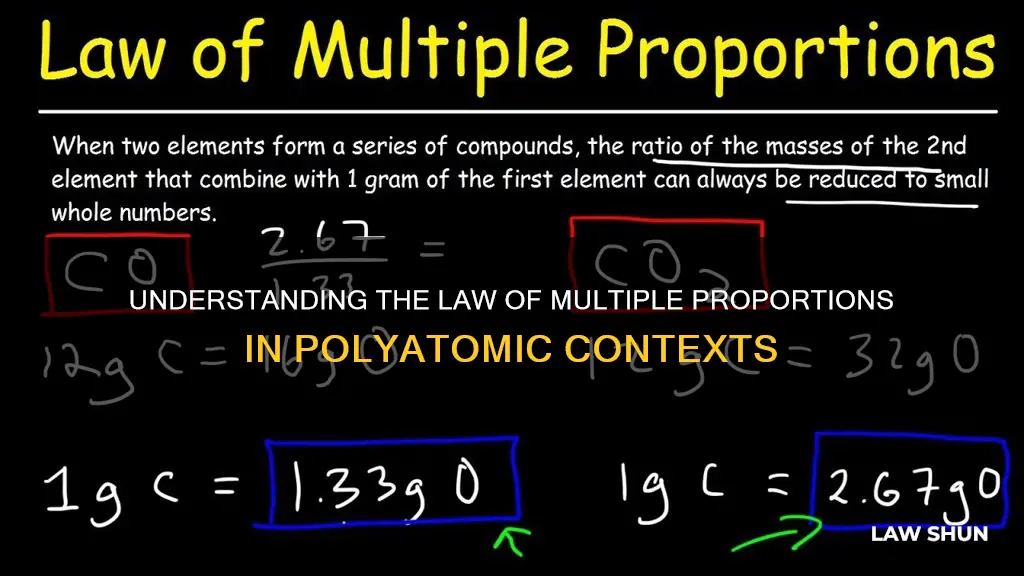
The law of multiple proportions, also known as Dalton's Law, was discovered by English chemist John Dalton in 1803 or 1804. The law states that when two elements combine to form multiple compounds, the ratio of the weight of one element to a fixed weight of the other will be a ratio of small whole numbers. For example, in carbon monoxide (CO), 12 grams of carbon combines with 16 grams of oxygen, while in carbon dioxide (CO2), 12 grams of carbon combines with 32 grams of oxygen. The ratio of the masses of oxygen to carbon (16:32) simplifies to 1:2, a ratio of small whole numbers.
The discovery of this law led Dalton to develop the modern theory of atoms. He proposed that elements combine with each other in multiples of a basic quantity, suggesting that there are indivisible atoms that combine to form molecules. This theory was supported by the law of multiple proportions, as it showed that there are definite proportions in which elements combine, and these proportions can be expressed as whole-number ratios.
Dalton's atomic theory gained widespread interest shortly after he published it, but it was not universally accepted at first. This was because the law of multiple proportions alone was not sufficient proof of the existence of atoms. However, over the course of the 19th century, further discoveries in chemistry and physics provided additional support for atomic theory, leading to its universal acceptance by the end of the century.
The law of multiple proportions forms the basis of stoichiometry and is considered one of the pathways for framing modern chemistry. It is applicable to simple compounds but often does not hold for large molecules or complex compounds, such as polymers and oligomers. Nonetheless, it is a fundamental concept in chemistry, helping us understand the constituents and ratios of elements in compounds.
CAS and Law School Applications: Are They Necessary?
You may want to see also
Frequently asked questions
The law of multiple proportions states that when two elements combine to form two or more compounds, the ratio of the masses of one element that combines with a fixed mass of the other are in a ratio of small whole numbers.
The law of multiple proportions is efficiently applicable to simple compounds. It cannot be applied to non-stoichiometric compounds, polymers, or oligomers.
The law of multiple proportions can be applied to polyatomic compounds in the same way as simple compounds. However, it is important to note that the law is most accurate for simple compounds and may not hold true for very large molecules.







