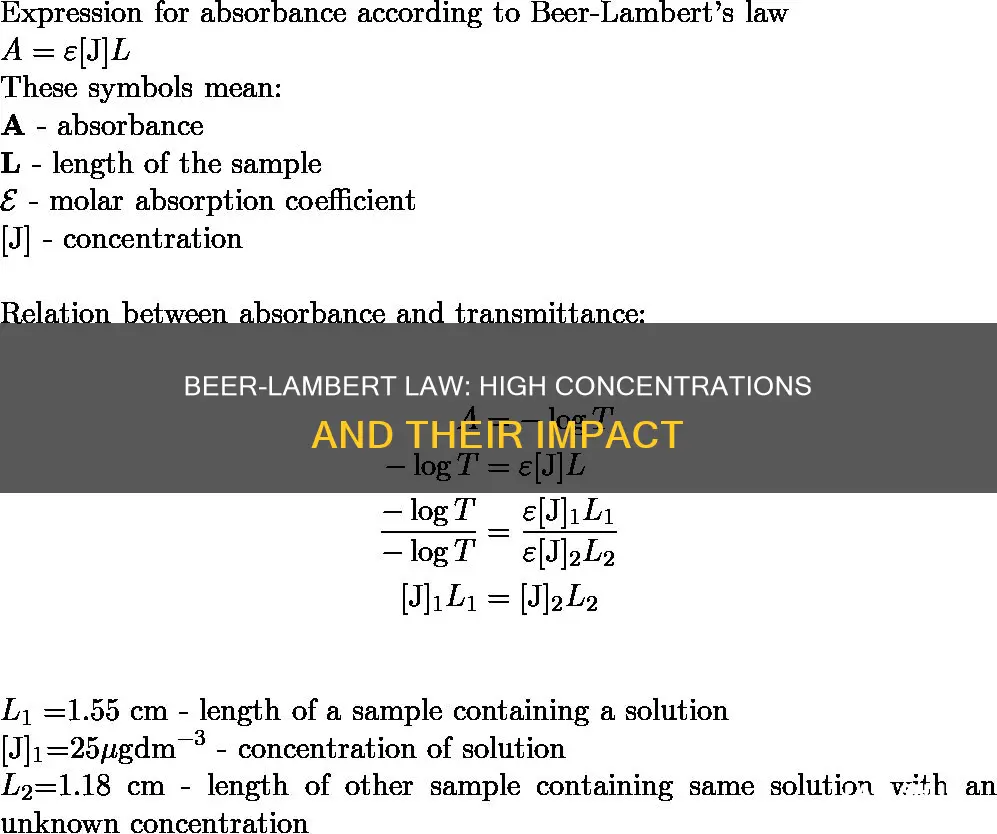
The Beer-Lambert law is an empirical relationship that describes the attenuation in intensity of a radiation beam passing through a macroscopically homogenous medium. The law states that the intensity of radiation decays exponentially in the absorbance of the medium, and that said absorbance is proportional to the length of the beam passing through the medium, the concentration of interacting matter along that path, and a constant representing the matter's propensity to interact.
The Beer-Lambert law is widely applied in chemical analysis and physical optics. However, it tends to break down at very high concentrations, especially if the material is highly scattering. This is because the concentration dependence is generally non-linear, and the law is only valid under certain conditions. At high concentrations, the proximity between the molecules of the solution is so close that there are deviations in the absorptivity. Additionally, when the concentration is high, the refractive index changes.
| Characteristics | Values |
|---|---|
| Concentration | High concentrations of a particular metallic solution do not follow Beer's Law |
| Radiation | At high concentrations, the analyte begins to behave differently due to interactions with the solvent and other solute molecules |
| Self-quenching | At high concentrations, a part of the absorbed radiation will be emitted back by self-quenching |
| Lambert Beer Law | At high concentrations, the Lambert Beer Law cannot give good correlations because when the absorbance is higher than 1, all light is absorbed |
| Precision | At high concentrations, the ratio I / I_0 approaches 0, so hardly any light is transmitted |
| Sensitivity | At high concentrations, problems with instrument sensitivity are encountered |
| Inner filter effect | At high concentrations, the inner filter effect introduces deviations |
| Association | At high concentrations, the analyte molecules may undergo association, dissociation and interaction with the solvent to produce a product with different absorption characteristics |
| Aggregation | At high concentrations, the solute may undergo aggregation, which will affect the extinction coefficient |
| Dehydration | At high concentrations, the solute may undergo dehydration, which will affect the extinction coefficient |
| Polychromicity of radiation | At high concentrations, the polychromicity of the radiation causes a negative deviation from Beer's Law |
What You'll Learn

High concentrations of a particular metallic solution
Beer's Law, also known as the Beer-Lambert law, states that the attenuation of a radiation beam passing through a medium is directly proportional to the length of the beam in the medium and the concentration of the interacting matter along that path.
However, this law does not hold in the case of high concentrations of a particular metallic solution. This is because, at high concentrations, the absorbance is higher than 1, meaning that all light is absorbed. Under these conditions, the absorbance is not correct.
Additionally, at high concentrations, the analyte molecules may begin to interact with each other, altering their ability to absorb radiation. This effect can also lead to a negative deviation from Beer's Law.
Furthermore, at high concentrations, the refractive index of the solution may be altered, affecting the absorbance obtained.
Therefore, Beer's Law breaks down at high concentrations of a particular metallic solution due to incorrect absorbance values and deviations caused by interactions between analyte molecules and changes in the refractive index of the solution.
Did SBF Break the Law?
You may want to see also

Limitations of Beer-Lambert Law
The Beer-Lambert law is an empirical relationship that describes the attenuation in the intensity of a radiation beam passing through a macroscopically homogeneous medium. It states that the intensity of radiation decays exponentially in the absorbance of the medium, and that this absorbance is proportional to the length of the beam passing through the medium, the concentration of interacting matter along that path, and a constant representing the matter's propensity to interact.
However, the Beer-Lambert law has its limitations and is not always applicable. These limitations can be classified into three categories: fundamental, chemical, and instrumental.
Fundamental Limitations
The Beer-Lambert law is a limiting law that is valid only for low concentrations of analyte. This is because, at higher concentrations, the individual particles of analyte are no longer independent of each other, and their proximity can cause deviations in absorptivity. Additionally, the analyte's absorptivity depends on the solution's refractive index, which can change with concentration, further affecting the accuracy of the Beer-Lambert law.
Chemical Limitations
Chemical deviations from the Beer-Lambert law may occur if the analyte is involved in an equilibrium reaction. For example, if the analyte is a weak acid, HA, which is in equilibrium with its conjugate weak base, A–, the Beer-Lambert law will not hold true as the acid and base forms may have different absorptivities.
Instrumental Limitations
There are also two principal instrumental limitations to the Beer-Lambert law. Firstly, the law assumes that the radiation reaching the sample is of a single wavelength, i.e., it assumes a purely monochromatic source of radiation. However, even the best wavelength selectors pass radiation with a small but finite effective bandwidth, leading to a negative deviation from the Beer-Lambert law.
Secondly, stray radiation can arise from imperfections in the wavelength selector, allowing light to reach the detector without passing through the sample. This results in an absorbance that is smaller than expected, particularly at higher concentrations, leading to another negative deviation from the Beer-Lambert law.
Marjorie Taylor Greene: Lawbreaker or Law-abiding?
You may want to see also

Instrumental factors
- Stray Radiation: The presence of stray radiation, even in small amounts, can affect the accuracy of measurements, especially at high concentrations. This is because the stray radiation adds to the detected signal, causing a negative deviation from Beer's Law.
- Polychromatic Radiation: Using radiation that is not purely monochromatic can also lead to deviations, especially at high concentrations. This is because the sample may have different molar absorptivities for different wavelengths of radiation, and the total absorbance is no longer linear with concentration.
- Slit Width: The width of the slit in the monochromator affects the amount of monochromatic radiation reaching the sample. A narrower slit width reduces the amount of polychromatic radiation, improving the linearity of Beer's Law but reducing the overall power of radiation (Po). Therefore, there is a trade-off between the desire for high source power and the desire for high monochromaticity.
- Detector Sensitivity: At very high concentrations, the amount of light transmitted through the sample becomes extremely low, and the detector's sensitivity may not be sufficient to accurately measure the transmitted light.
- Interference Effects: Interference effects, such as the "electric field standing wave effect," can alter the simple relationship between the dielectric function and absorption index, invalidating Beer's Law.
- Scattering: In some cases, scattering of radiation can occur within the sample or due to the sample container. Scattering can reduce the intensity of radiation reaching the detector and lead to deviations from Beer's Law.
These instrumental factors highlight the importance of careful experimental design and the use of appropriate equipment to ensure accurate measurements when applying Beer's Law.
Antisocial Personality Disorder: Criminals or Misunderstood?
You may want to see also

Non-linearity in absorbance
At high concentrations, the absorbance can exceed 1, indicating that all the light has been absorbed. In such cases, the measured absorbance is not accurate, and the Beer-Lambert law fails to provide good correlations. Additionally, high concentrations can lead to interactions between solute molecules, altering their charge distribution and absorption behaviour. This can result in a shift in the absorption wavelength and a non-linear relationship between absorbance and concentration.
At low concentrations, the difference between the transmitted and incident light intensities becomes very small, approaching a ratio of 1. This can lead to significant errors in the measured absorbance, affecting the precision of the absorbance measurement.
The non-linearity in absorbance at high concentrations can be further attributed to various factors. For instance, the analyte molecules may undergo association, dissociation, or interaction with the solvent, resulting in a product with different absorption characteristics. Additionally, high concentrations can lead to self-quenching, where a part of the absorbed radiation is emitted back due to collisions, resulting in erroneous absorbance values.
Instrumental factors can also contribute to non-linearity. For example, the use of polychromatic radiation instead of purely monochromatic radiation can lead to deviations from Beer's law. This is because the net absorbance added over all wavelengths is no longer linear with concentration, resulting in a negative deviation at higher concentrations.
Furthermore, the presence of stray radiation or background noise in the instrument can affect the accuracy of absorbance measurements, especially at high concentrations. As the concentration increases, the amount of radiation reaching the detector decreases, and the contribution of stray radiation becomes more significant, leading to a negative deviation from Beer's law.
To summarise, non-linearity in absorbance occurs when the Beer-Lambert law breaks down at high and low concentrations of a solute. This can be due to chemical interactions, instrumental limitations, or the inherent non-linear nature of the concentration dependence.
Iraq War: International Law Violation or Justification?
You may want to see also

The role of stray radiation
Stray radiation is a term used to refer to the small amount of radiation that leaks into a detector. This can affect the accuracy of measurements, particularly at higher concentrations.
In the context of Beer's Law, the presence of stray radiation can impact the linearity of the relationship between absorbance and concentration. Beer's Law assumes that the molecules absorbing radiation do not interact with each other and that the incident radiation is purely monochromatic. However, in reality, there may be a small amount of stray radiation that reaches the detector along with the incident radiation. This stray radiation can be considered a constant or fixed quantity.
When stray radiation is taken into account, the expression for absorbance can be modified to include terms for both the incident radiation and the stray radiation:
Expression for Absorbance Including Stray Radiation:
> A = log((Po + Ps) / (P + Ps))
Where:
- A = Absorbance
- Po = Power from the radiation source
- Ps = Power of stray radiation
- P = Radiation reaching the detector
At low concentrations, the effect of stray radiation is negligible because the amount of radiation absorbed is small, and P is still much greater than Ps. However, as the concentration increases, P decreases, and the denominator (P + Ps) approaches the constant value of Ps. This results in the absorbance approaching a constant value rather than increasing linearly. This deviation from linearity at higher concentrations is known as a negative deviation from Beer's Law.
To minimise the impact of stray radiation and maintain the linearity of Beer's Law, it is essential to reduce the amount of stray radiation reaching the detector. This can be achieved by using appropriate instrumentation and ensuring that the experimental setup minimises any potential sources of stray radiation. Additionally, the use of monochromatic radiation, as assumed in Beer's Law, can help reduce deviations caused by stray radiation.
Understanding Employee Break Rights and Federal Law
You may want to see also
Frequently asked questions
Beer's Law fails at higher concentrations because the linearity of the law is limited to chemical and instrumental factors. When the solution has higher concentrations, the proximity between the molecules of the solution is so close that there are deviations in the absorptivity. Also, when the concentration is high, the refractive index changes.
Beer's Law has other limitations, including:
- A diluted solution is used
- There shouldn't be a scattering of the light beam
- Monochromatic electromagnetic radiation should be used
Beer's Law for absorption spectroscopy is a linear relationship between the absorbance and the concentration of an absorbing species. The states imply that the type, as well as the concentration of the molecules, are necessary.
Beer's Law is not obeyed in the following situations:
- When different types of molecules are in equilibrium with each other
- An association complex is formed by the solute and the solvent
- When fluorescent compounds are used
- When thermal equilibrium is attained between the excited state and the ground state







