Boyle's law, also known as Mariotte's law, is a fundamental principle in chemistry that describes the behaviour of gas held at a constant temperature. The law, formulated by Anglo-Irish chemist Robert Boyle in 1662, states that the pressure exerted by a gas is inversely proportional to the volume it occupies, as long as the temperature and the quantity of gas remain constant. This relationship can be expressed mathematically as PV = K, where P is the pressure, V is the volume, and K is a constant. This law applies to gases at low pressures, and deviations from ideal gas behaviour are observed at higher pressures. While the law is stated in millibars (mbar), it is unclear if it applies when measuring pressure in mbar specifically.
What You'll Learn

Pressure and volume are inversely proportional
Boyle's law was formulated by Anglo-Irish chemist Robert Boyle in 1662, although French physicist Edme Mariotte also discovered the relationship in 1676 (or 1679). The law is a basic principle in chemistry, describing the behaviour of a gas held at a constant temperature.
Boyle's law can be observed in several real-world applications. For example, when blowing up a balloon, the pressure of the air inside pushes on the rubber, causing the balloon to expand. Squeezing one end of the balloon increases the internal pressure, causing the other end to expand outward. Eventually, the pressure becomes so great that it causes the balloon to burst.
Another example of Boyle's law in action is the expansion of gas inside a scuba diver's body when ascending from a deep dive. As the diver ascends, the surrounding pressure decreases, causing the gas molecules in their body to expand. This expansion can be harmful and even fatal if the diver ascends too quickly.
Boyle's law also explains the phenomenon of soda bottles foaming when opened too quickly. The soda is pumped into the bottle by passing it through carbon dioxide, and when the bottle is opened, the pressure on the gas is reduced, causing its volume to expand and rush out of the bottle.
HIPAA Laws and Vaccines: What's the Connection?
You may want to see also

The law applies to low pressure, not high pressure
Boyle's Law, also known as the Boyle-Mariotte Law, is a fundamental law in chemistry that describes the behaviour of a gas held at a constant temperature. The law was discovered by Robert Boyle in 1662 and states that at a fixed temperature, the volume of gas is inversely proportional to the pressure exerted by the gas. In other words, when the volume of a gas decreases, its pressure increases, and vice versa. This relationship between pressure and volume can be expressed mathematically as PV=k, where P is the pressure, V is the volume, and k is a constant.
Boyle's Law applies to low-pressure situations and not high-pressure ones. This is because, at high pressures, gases behave like ideal gases. The technology available at the time of the law's discovery in the 17th century could not produce very high pressures or very low temperatures, so deviations from ideal gas behaviour at high pressures were not observed. However, as technology improved and higher pressures and lower temperatures became achievable, it became apparent that the relationship between pressure and volume at high pressures could not be accurately described by Boyle's Law.
The law can be applied to various real-life situations, such as spray paint cans, soda bottles, and deep-sea diving. For example, when you open a soda bottle, you are reducing the pressure on the gas inside, which causes the volume of the gas to expand and rush out of the bottle. Similarly, when a diver returns to the surface of the water, the decrease in pressure causes the gas molecules in their body to expand, which can be dangerous and even fatal.
Boyle's Law is significant because it was the first law to explain the behaviour of gases and it forms the basis for understanding gas laws. It also has practical applications in various fields, such as chemistry, physics, and scuba diving.
Are Executives Exempt From Claim Adjuster License Laws?
You may want to see also

The law was discovered by Robert Boyle in 1662
Robert Boyle (1627-1691) was an Anglo-Irish natural philosopher, chemist, physicist, alchemist, and inventor. He is regarded as the first modern chemist and, thus, one of the founders of modern chemistry and pioneers of modern experimental scientific methods. Boyle was born in Lismore Castle, County Waterford, Ireland, as the 14th child of the Earl of Cork. As a young man, he was tutored at home and on the Continent. He spent the later years of the English Civil Wars at Oxford, where he read and experimented with his assistants and colleagues.
Boyle was a leading scientist and intellectual of his day and a great proponent of the experimental method. He was committed to the New Philosophy, which valued observation and experiment at least as much as logical thinking in formulating accurate scientific understanding. He played a key role in founding the Royal Society to nurture this new view of science.
Boyle's first published scientific work, 'New Experiments Physico-Mechanicall, Touching the Spring of the Air, and Its Effects' (1660), concerned the physical nature of air. In it, he used an air pump to create a vacuum. The second edition of this work, published in 1662, delineated the quantitative relationship that Boyle derived from experimental values, later known as Boyle's Law: the volume of a gas varies inversely with pressure.
Boyle was an advocate of corpuscularism, a form of atomism that was slowly displacing Aristotelian and Paracelsian views of the world. Instead of defining physical reality and analyzing change in terms of Aristotelian substance and form and the four classical elements, or the three Paracelsian elements, corpuscularism discussed reality and change in terms of particles and their motion.
Boyle believed that chemical experiments could demonstrate the truth of the corpuscularian philosophy. In this context, he defined elements as "certain primitive and simple, or perfectly unmingled bodies; which not being made of any other bodies, or of one another, are the ingredients of which all those called perfectly mixt bodies are immediately compounded, and into which they are ultimately resolved."
Boyle carried out the principles espoused by Francis Bacon in the 'Novum Organum'. He refrained from studying atomical and Cartesian systems and even Bacon's 'Novum Organum' itself, though he admitted to "transiently consulting" them about a few particulars. Nothing was more alien to his mental temperament than the spinning of hypotheses. He regarded the acquisition of knowledge as an end in itself, and consequently, he gained a wider outlook on the aims of scientific inquiry than his predecessors.
Boyle was also interested in theology, showing a leaning towards the practical side and an indifference to controversial polemics. He incorporated his scientific interests into his theology, believing that natural philosophy could provide powerful evidence for the existence of God. He attempted to tackle complex theological questions using methods derived from his scientific practices. Throughout his career, Boyle tried to show that science could lend support to Christianity.
Space Laws: Do Legal Boundaries Extend Beyond Earth?
You may want to see also

It is the first law to describe the behaviour of gases
Boyle's Law, also known as the Boyle-Mariotte Law, is widely regarded as the first law to describe the behaviour of gases. It was formulated by Anglo-Irish chemist Robert Boyle in 1662 and published in the work entitled "The Sceptical Chymist".
The law describes the behaviour of a gas confined within a closed system, stating that the pressure exerted by a given mass of an ideal gas is inversely proportional to the volume it occupies, as long as the temperature and the amount of gas remain constant.
Mathematically, this can be expressed as:
P ∝ (1/V)
Or
P x V = k
Where P is the pressure of the gas, V is the volume of the gas, and k is a constant for a particular temperature and amount of gas.
In other words, if the temperature and the amount of gas remain constant, an increase in pressure will result in a decrease in volume, and vice versa. This relationship was also independently discovered by French physicist Edme Mariotte in 1676 or 1679, after Boyle's original publication.
Discrimination Laws: Contractors' Rights and Legal Protection
You may want to see also

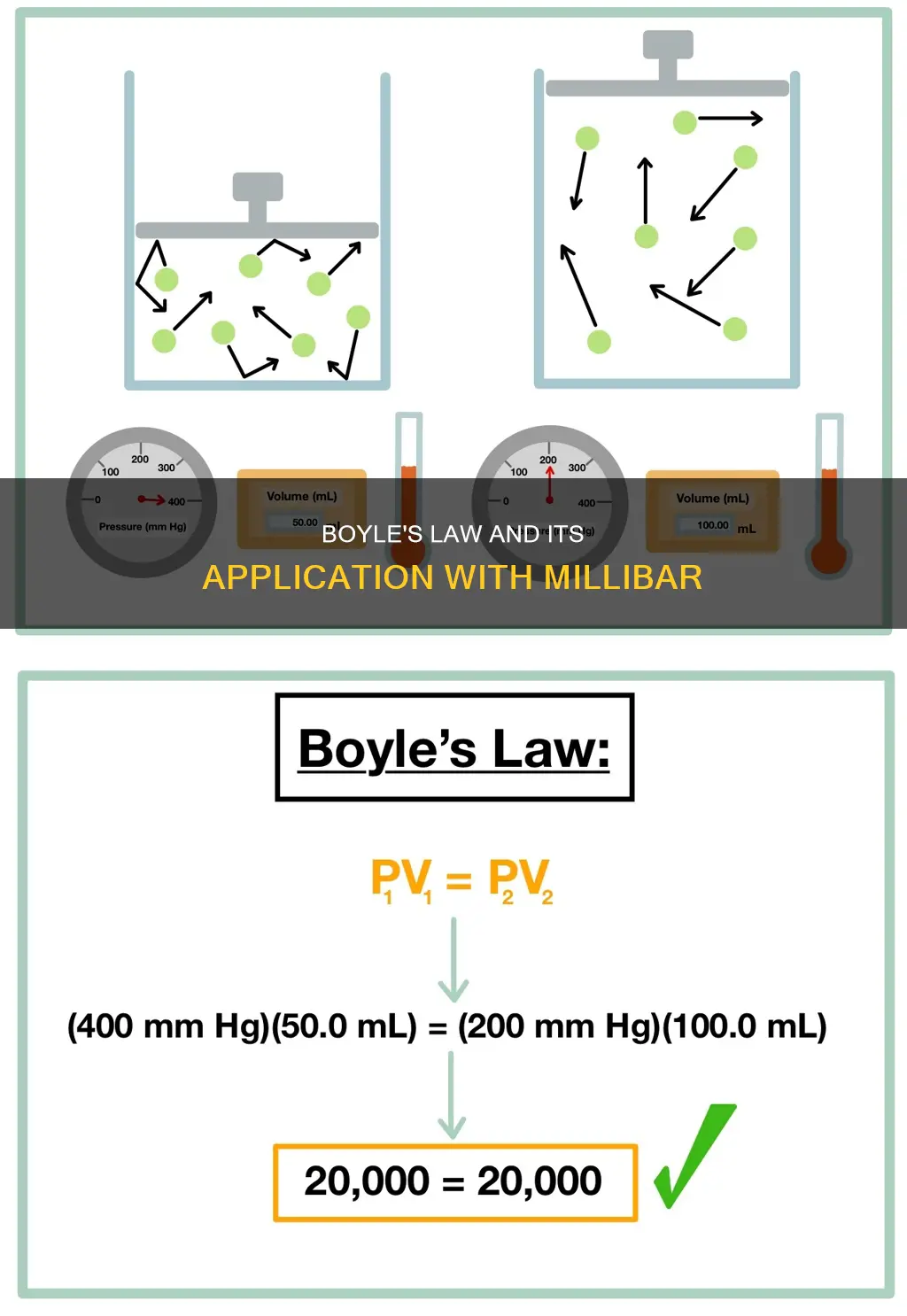
The mathematical equation for Boyle's Law is PV=K
The mathematical equation for Boyle's Law is indeed PV=K. In this equation, P denotes the pressure of a system, V denotes the volume of a gas, and K is a constant value representative of the temperature of the system and the amount of gas.
Boyle's Law, also known as the Boyle-Mariotte Law or Mariotte's Law, is an empirical gas law that describes the relationship between the pressure and volume of a confined gas. The law states that the pressure exerted by a gas (of a given mass) is inversely proportional to the volume it occupies, as long as the temperature and amount of gas remain unchanged within a closed system. This can be expressed mathematically as P ∝ (1/V).
The proportionality can be converted into an equation by adding a constant, K. This constant is representative of the temperature of the system and the amount of gas. The equation then becomes PV=K.
The initial and final volumes and pressures of a fixed amount of gas, where the initial and final temperatures are the same, are related by the equation:
P1V1=P2V2
Here, P1 and V1 represent the original pressure and volume, and P2 and V2 represent the second pressure and volume. This equation can be used to predict the result of introducing a change in volume and pressure to the initial state of a fixed quantity of gas. For example, if the volume of a gas is halved, the pressure is doubled, and vice versa.
The Law and Black People: A Complex History
You may want to see also
Frequently asked questions
Boyle's Law is a gas law that describes the relationship between the pressure exerted by a gas and the volume it occupies. The law states that when the temperature and the amount of gas remain constant, the pressure and volume are inversely proportional.
Boyle's Law can be calculated using the equation: PV = k, where P is the pressure of the gas in mbar, V is the volume of the gas, and k is a constant.
The pressure (P) is measured in pascals (Pa), atmospheres (atm), or millibars (mbar). The volume (V) is measured in litres (L) or cubic metres (m^3). The constant (k) has no units as it is the product of pressure and volume.
Boyle's Law has various real-life applications, including spray paint or aerosol cans, opening a carbonated soda bottle, and deep-water diving. In each case, the change in pressure causes a change in the volume of the gas, which can have visible effects like the release of paint or soda, or dangerous consequences for divers if they ascend too quickly.







