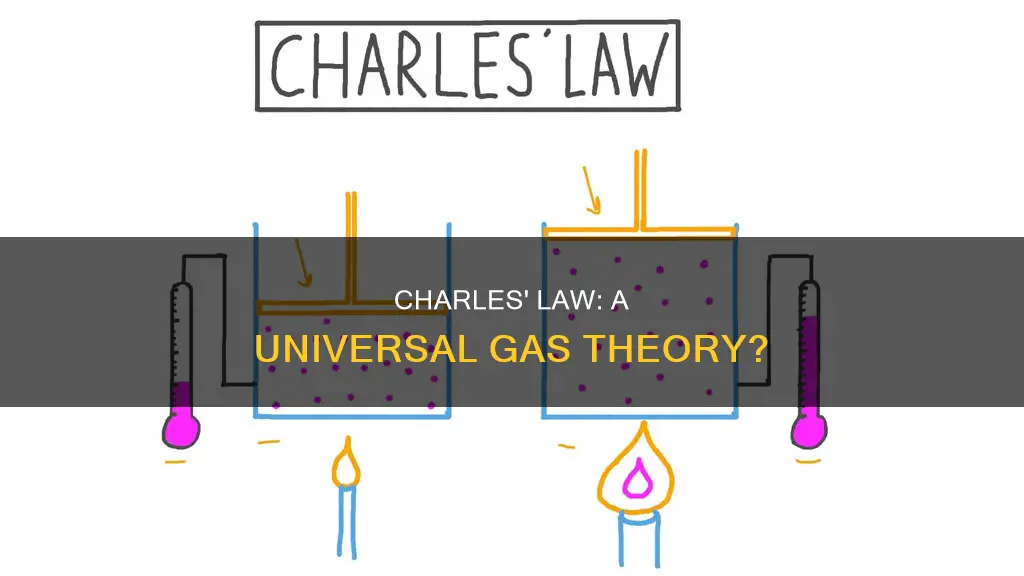
John Dalton was the first to demonstrate that Charles's Law, which states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept constant, applied generally to all gases. This was later confirmed by French natural philosopher Joseph Louis Gay-Lussac. However, Gay-Lussac was unable to show that the equation relating volume to temperature was a linear function. Charles's Law can be written as:
> V/T = k
Where V is the volume of the gas, T is the temperature of the gas (measured in Kelvins), and k is a constant for a particular pressure and amount of gas.
| Characteristics | Values |
|---|---|
| What is Charles' Law? | Charles' Law states that the volume of a gas is directly proportional to its absolute temperature when pressure is kept constant. |
| Mathematical representation | V/T = k, where V is volume, T is temperature, and k is a constant. |
| Applicability | The law applies generally to all gases, and to the vapours of volatile liquids if the temperature is well above the boiling point. |
| Ideal gas | An ideal gas adheres to the equation of state pV = nRT regardless of its pressure, volume, and temperature. |
| Temperature scale | The Kelvin scale must be used because zero on the Kelvin scale corresponds to a complete stoppage of molecular motion. |
| Relation to absolute zero | Charles' Law appears to imply that the volume of a gas will descend to zero at a certain temperature (−266.66 °C according to Gay-Lussac's figures) or −273.15 °C. |
What You'll Learn
- Charles's Law states that the volume of a gas is directly proportional to its temperature when pressure is kept constant
- The law can be used to compare changing conditions for a gas
- It can be used to calculate the unknown volume of a gas
- The law helped define the concept of absolute zero
- Charles's Law applies to all gases

Charles's Law states that the volume of a gas is directly proportional to its temperature when pressure is kept constant
Charles's Law, also known as the Law of Volumes, is an experimental gas law that describes how gases tend to expand when heated. The law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept constant.
The French physicist Jacques Charles studied the effect of temperature on the volume of a gas at constant pressure in the 1780s. He found that the volume of a gas is directly proportional to its temperature. This relationship can be expressed mathematically as:
> V ∝ T
Or
> V = kT
Where V is the volume of the gas, T is the temperature of the gas (measured in Kelvins), and k is a constant for a particular pressure and amount of gas.
This law can also be used to compare the same substance under two different sets of conditions, and the equation becomes:
> V1/T1 = V2/T2
This equation shows that as the absolute temperature increases, the volume of the gas also increases proportionally.
Charles's Law is one of the gas laws, which are a set of fundamental principles that describe the behaviour of gases under different conditions such as pressure, temperature, and volume. These laws only apply to gases because gases have different interactions between their components compared to liquids and solids. Gases are highly compressible and are not bound together by strong intermolecular forces, allowing them to move freely and independently of each other.
Charles's Law is particularly useful for understanding real-world phenomena, such as the behaviour of gases in weather systems, the operation of internal combustion engines, and the function of the respiratory system in humans.
Florida Fertilizer Laws: Homeowner Compliance Requirements
You may want to see also

The law can be used to compare changing conditions for a gas
Charles's Law can be used to compare the changing conditions for a gas. The law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept constant. The absolute temperature must be measured in Kelvin as zero on the Kelvin scale corresponds to a complete stoppage of molecular motion.
To compare changing conditions, we use $V_1$ and $T_1$ to stand for the initial volume and temperature of a gas, and $V_2$ and $T_2$ to stand for the final volume and temperature. The mathematical relationship of Charles's Law becomes:
$$\frac{V_1}{T_1} = \frac{V_2}{T_2}$$
This equation can be used to calculate any one of the four quantities if the other three are known. The direct relationship will only hold if the temperatures are expressed in Kelvin.
For example, let's say we have a balloon with a volume of 2.20 L at a temperature of 22°C. If we then heat the balloon to a temperature of 71°C, we can use Charles's Law to find the new volume of the balloon. First, we rearrange the equation to solve for $V_2$:
$$V_2 = \frac{V_1 \times T_2}{T_1}$$
Now, we substitute the known quantities into the equation and solve:
$$V_2 = \frac{2.20 \text{ L} \times 344 \text{ K}}{295 \text{ K}} = 2.57 \text{ L}$$
So, the volume of the balloon increases as the temperature increases.
The Consciousness Conundrum: Physics Laws Applicable?
You may want to see also

It can be used to calculate the unknown volume of a gas
Charles's Law, also known as the law of volumes, describes the relationship between the volume of a gas and its temperature when the pressure and the mass of the gas are constant. It states that the volume is directly proportional to the absolute temperature.
The law can be written as:
> V1/T1 = V2/T2
Where V1 and T1 are the initial volume and temperature, and V2 and T2 are the final volume and temperature.
This equation shows that as the absolute temperature increases, the volume of the gas also increases proportionally.
To calculate the unknown volume of a gas using Charles's Law, you need to know three of the four parameters (V1, T1, V2, and T2) and can then calculate the fourth. For example, if you want to find the final volume, the formula becomes:
> V2 = V1 / T1 x T2
Similarly, if you want to find the final temperature, the formula becomes:
> T2 = T1 / V1 x V2
It's important to note that Charles's Law only applies to ideal gases, which are gases that follow the ideal gas law equation. Additionally, it is most applicable to low-molecular-weight or high-temperature gases.
Employment Laws: Tribal Governments and Job Rights
You may want to see also

The law helped define the concept of absolute zero
Charles's Law is a fundamental principle that describes the behaviour of gases under varying conditions of pressure, temperature, and volume. The law states that the volume of a gas is directly proportional to its absolute temperature when the pressure is kept constant. This relationship can be expressed mathematically as V/T = k, where V is volume, T is temperature, and k is a constant.
Charles's Law played a crucial role in establishing the concept of absolute zero, which is the temperature at which the volume of a gas theoretically becomes zero. By conducting experiments that measured the volume of a gas at different temperatures, scientists could use Charles's Law to extrapolate and determine the temperature at which the gas volume would reach zero. This temperature is known as absolute zero.
The law's direct relationship between volume and temperature, with pressure held constant, provided a reliable framework for these experiments. By measuring the volume of a gas at different temperatures and plugging those values into the equation V = a⋅ T + b, scientists could calculate absolute zero by interpreting it as the temperature (T0) at which gas volume becomes zero.
For example, in an experiment using an Erlenmeyer flask, the volume of gas at a high temperature (Vh) and a low temperature (Vl) was measured, along with the corresponding temperatures (Th and Tl). By using these values in the equation and solving for T0, scientists could estimate the temperature of absolute zero. This experimental approach, guided by Charles's Law, helped define the concept of absolute zero and provided valuable insights into the behaviour of gases at extremely low temperatures.
Benford's Law: Universal Truth or Mathematical Myth?
You may want to see also

Charles's Law applies to all gases
Charles's Law, also known as the Law of Volumes, is a gas law that applies to all gases. It describes the relationship between the volume and temperature of a gas when pressure is kept constant. The law states that the volume of a given mass of gas is directly proportional to its absolute temperature. This means that as the temperature of a gas increases, so does its volume, and vice versa.
Mathematically, Charles's Law can be expressed as:
$$\frac{V}{T} = k$$
Where:
- V is the volume of the gas
- T is the absolute temperature of the gas in Kelvin
- K is a constant for a particular pressure and amount of gas
This law is useful for understanding how gases behave when heated or cooled. For example, if a gas is heated while keeping the pressure constant, its volume will increase proportionally to the change in temperature. Similarly, if the temperature decreases, the volume of the gas will also decrease proportionally.
Charles's Law was formulated by French physicist Jacques Charles in the 1780s. It is one of the experimental gas laws, along with Boyle's Law and the Pressure Law, that describe the behaviour of ideal gases. These laws are based on the assumption that gases behave in certain ways, such as having no intermolecular interactions and occupying a negligible volume. While these assumptions may not hold true for all gases in all situations, Charles's Law and the other gas laws provide valuable insights into gas behaviour and have practical applications in various fields, including weather systems, internal combustion engines, and human respiratory systems.
Zoning Laws: Do They Affect Your Web Store?
You may want to see also