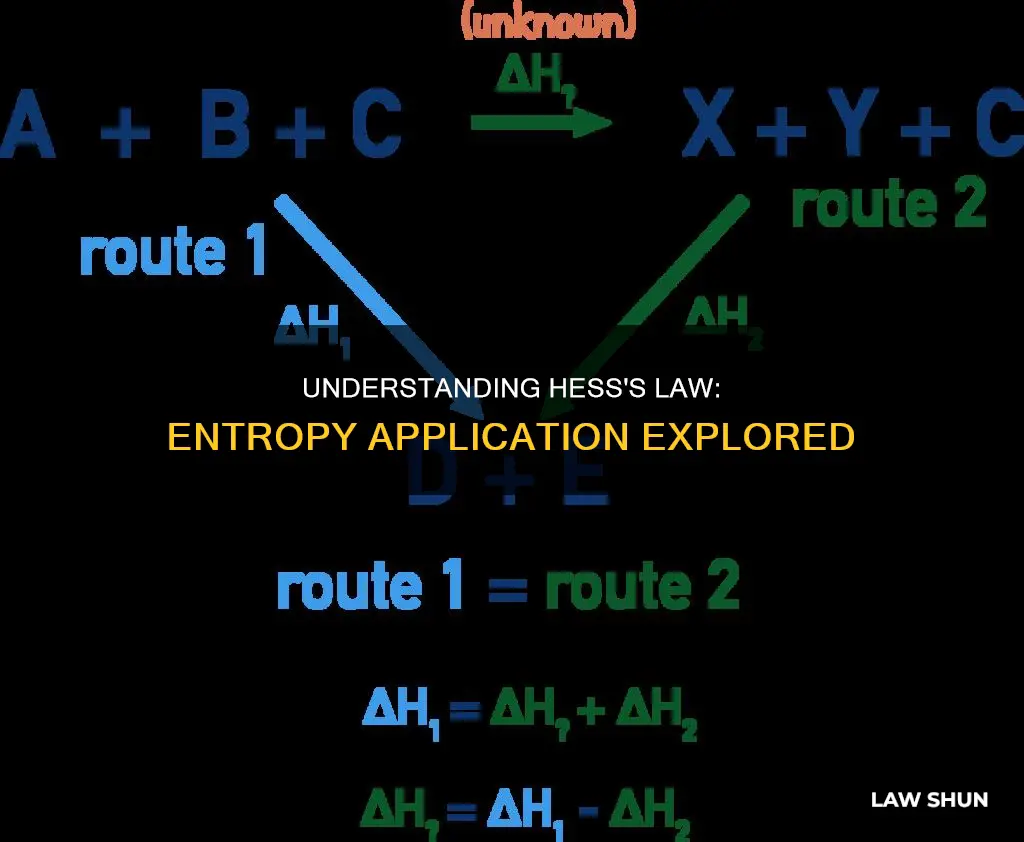
Hess's Law, also known as Hess's Law of Constant Heat Summation, is a principle in physical chemistry that applies to the total enthalpy change during a chemical reaction. It states that the overall enthalpy change is independent of the sequence of steps taken and is instead dependent on the initial and final states of the reactants and products. This law is derived from the first law of thermodynamics and is attributed to Swiss-born Russian chemist and physician Germain Hess, who formulated it in 1840. Hess's Law can be applied to calculate the overall change in enthalpy by summing up the changes for each step of a reaction, as long as the reactions occur under constant pressure and temperature. While Hess's Law is primarily associated with enthalpy, it can also be extended to include changes in entropy and Gibbs free energy, as these are also state functions.
| Characteristics | Values |
|---|---|
| Name | Hess's Law of Constant Heat Summation |
| Other Names | Hess's Law |
| Named After | Germain Hess, a Swiss-born Russian chemist and physician |
| Law States | The total enthalpy change during the complete course of a chemical reaction is independent of the sequence of steps taken |
| Application | Can be used to determine the overall energy required for a chemical reaction that can be divided into synthetic steps that are individually easier to characterize |
| Enthalpy | Enthalpy is an extensive property, meaning that its value is proportional to the system size |
| Enthalpy Change | Proportional to the number of moles participating in a given reaction |
| Enthalpy Calculation | ΔH° = ΣΔHn, where ΔH° is the heat absorbed or evolved and ΣΔHn is the sum of the heat absorbed or evolved in the individual n steps of the reaction |
| Requirements | Each equation must be correctly balanced, and all steps of the reaction must start and end at constant temperatures and pressures |
| Extension | Concepts can be expanded to include changes in entropy and in Gibbs free energy, since these are also state functions |
What You'll Learn

Hess's Law and Entropy Calculations
Hess's Law, also known as Hess's Law of Constant Heat Summation, is a principle in physical chemistry that applies to the calculation of enthalpy changes in a reaction. It states that the total enthalpy change in a chemical reaction is independent of the sequence of steps taken and remains the same whether the reaction occurs in one step or multiple steps. This is because enthalpy is a state function, and its value is dependent only on the initial and final states of the reactants and products, regardless of the path taken.
Mathematically, Hess's Law can be expressed as:
> ΔH° = ΣΔHn
Where ΔH° is the heat absorbed or released, and ΣΔHn represents the sum of the heat absorbed or released in each of the 'n' steps of the reaction.
Hess's Law is useful for calculating the enthalpy changes in reactions that cannot be measured directly. This is achieved by performing algebraic operations on the chemical equations of reactions using previously determined values for the enthalpies of formation. The law requires that each step of a multi-step reaction is correctly balanced and occurs at constant temperatures and pressures.
While Hess's Law is primarily associated with enthalpy changes, it can also be applied to other state functions, including entropy and Gibbs free energy calculations. Entropy represents the level of disorder in a system, and its change in a reaction can be determined using Hess's Law by summing the absolute entropies of the products and subtracting the absolute entropies of the reactants:
> ΔS = ΣS(products) - ΣS(reactants)
This application of Hess's Law assumes that the entropy values are provided in absolute terms, rather than relative to the elements in their reference states, as is the case with enthalpy.
In summary, Hess's Law provides a valuable tool for calculating enthalpy changes in complex reactions by breaking them down into simpler steps. Additionally, it can be extended to determine entropy and Gibbs free energy changes, making it a versatile concept in thermodynamics and chemistry.
HIPAA Laws: Employee Rights and Responsibilities Explained
You may want to see also

Hess's Law and Enthalpy
Hess's Law, also known as the Law of Constant Heat Summation, is a fundamental principle in physical chemistry that was formulated by Swiss-born Russian chemist and physician Germain Hess in 1840. This law states that the total enthalpy change during a chemical reaction remains constant, regardless of the number of steps or the specific pathway taken. In other words, the overall enthalpy change is the same whether the reaction occurs in one step or multiple steps, as long as the initial and final states of the reactants and products are identical.
Hess's Law can be expressed as:
> ΔH° = ΣΔHn
Here, ΔH° represents the heat absorbed or released, and ΣΔHn denotes the sum of the heat changes in each of the 'n' individual steps of the reaction. This law is a direct consequence of the first law of thermodynamics and underscores the fact that enthalpy is a state function. As a result, the enthalpy change in a system due to a reaction at constant pressure is equal to the heat absorbed or released, which can be determined through calorimetry.
The significance of Hess's Law lies in its ability to determine the overall energy required for a complex chemical reaction by breaking it down into simpler, more manageable synthetic steps. This approach allows for the compilation of standard enthalpies of formation, which are invaluable for predicting enthalpy changes in intricate synthesis processes. Furthermore, Hess's Law enables the calculation of enthalpy changes even when direct measurement is not feasible. This is achieved by employing basic algebraic operations on the chemical equations of reactions, utilising previously established values for the enthalpies of formation.
The versatility of Hess's Law extends beyond enthalpy calculations. It can also be applied to determine changes in entropy and Gibbs free energy, as these are also state functions. For instance, the Bordwell thermodynamic cycle leverages Hess's Law principles to determine experimentally inaccessible Gibbs free energy values. By combining ΔG values from the Bordwell cycle with ΔH values obtained through Hess's Law, it becomes possible to calculate entropy values that have not been directly measured.
Limits and Laws: When One Limit Doesn't Exist
You may want to see also

Hess's Law: Thermodynamic Cycles
Hess's Law, also known as Hess's Law of Constant Heat Summation, is a principle in physical chemistry named after Swiss-born Russian chemist and physician Germain Hess. Hess formulated this law in 1840, and it states that the total enthalpy change during a chemical reaction is independent of the sequence of steps taken. In other words, the overall enthalpy change is the same regardless of the route by which the chemical change occurs, as long as the initial and final conditions are the same. This is because enthalpy is a state function, and the enthalpy change in a system at constant pressure is equal to the heat absorbed or released.
Hess's Law can be applied to thermodynamic cycles, which are a series of thermodynamic processes that eventually return a system to its initial state. These cycles can be used to determine the overall energy required for a chemical reaction that can be divided into multiple steps. By summing the enthalpy changes for each step of the cycle, Hess's Law allows for the calculation of the total enthalpy change, which should be zero for a complete cycle.
The law can be mathematically expressed as:
> net enthalpy change = ∆Hnet = ∑∆Hr
Where ∆Hnet is the net enthalpy change, and ∑∆Hr is the sum of the enthalpy changes for each step of the cycle.
Hess's Law is a valuable tool for understanding and predicting the behaviour of chemical reactions. It allows chemists to determine the overall energy requirements for complex reactions by breaking them down into simpler, more manageable steps. Additionally, it provides insight into the underlying principles of thermodynamics, specifically the first law of thermodynamics, which states that energy cannot be created or destroyed, only transferred or transformed.
Furthermore, Hess's Law can be extended beyond enthalpy changes to include changes in entropy and Gibbs free energy, as these are also state functions. This extension is exemplified by the Bordwell thermodynamic cycle, which utilises easily measured equilibria and redox potentials to determine experimentally inaccessible Gibbs free energy values. By combining ΔG values from the Bordwell cycle with ΔH values found using Hess's Law, it is possible to calculate entropy values that have not been directly measured.
Life Insurance and HIPAA: What's the Deal?
You may want to see also

Hess's Law and Gibbs Free Energy
Hess's Law, also known as Hess's Law of Constant Heat Summation, is a relationship in physical chemistry named after Germain Hess, a Swiss-born Russian chemist and physician who published it in 1840. The law states that the total enthalpy change during the complete course of a chemical reaction is independent of the sequence of steps taken. In other words, the change in enthalpy in a chemical reaction is the same regardless of whether the reaction takes place in one step or several steps, as long as the initial and final states of the reactants and products are the same. Enthalpy is an extensive property, meaning its value is proportional to the system size, and the enthalpy change is proportional to the number of moles participating in a given reaction.
Hess's Law is based on the fact that enthalpy is a state function, meaning it only depends on the initial and final states and not on how it was achieved. This is similar to altitude, which is also a state function. For example, two hikers can meet at a 500-foot altitude, but one may be on their way to the top, while the other is returning from a longer walk.
Hess's Law can be used to calculate the enthalpy change (ΔH) for a reaction even when the enthalpies of formation for some or all of the components are unknown. This is done by performing basic algebraic operations based on the chemical equations of reactions using previously determined values for the enthalpies of formation.
The concepts of Hess's Law can be extended to include changes in entropy and Gibbs free energy, as these are also state functions. For example, the Bordwell thermodynamic cycle uses easily measured equilibria and redox potentials to determine experimentally inaccessible Gibbs free energy values. By combining ΔG values from Bordwell thermodynamic cycles and ΔH values found with Hess's Law, it is possible to calculate entropy values that have not been directly measured.
For the free energy, the equation is as follows:
{\displaystyle \Delta G_{\text{reaction}}^{\ominus }=\sum \nu _{\text{p}}\Delta G_{\mathrm {f} \,({\text{p}})}^{\ominus }-\sum \nu _{\text{r}}\Delta G_{\mathrm {f} \,({\text{r}})}^{\ominus }.}
For entropy, the situation is slightly different. Entropy can be measured as an absolute value, not relative to those of the elements in their reference states (as with ΔH), so there is no need to use the entropy of formation. Instead, the absolute entropies for products and reactants are used:
{\displaystyle \Delta S_{\text{reaction}}^{\ominus }=\sum \nu _{\text{p}}S_{({\text{p}})}^{\ominus }-\sum \nu _{\text{r}}S_{({\text{r}})}^{\ominus}.}
In summary, Hess's Law is a powerful tool in chemistry that allows for the calculation of enthalpy changes in chemical reactions, even when direct measurement is not possible. Additionally, by combining Hess's Law with other thermodynamic cycles, it can be used to determine Gibbs free energy and entropy values, making it a versatile concept in chemical thermodynamics.
Leash Laws: Rental Property Rules and Regulations Explained
You may want to see also

Hess's Law: Determining Enthalpies
Hess's Law, also known as Hess's Law of Constant Heat Summation, was formulated by Germain Henri Hess, a Swiss-born Russian chemist and physician, in 1840. This law states that the total enthalpy change during a chemical reaction is independent of the sequence of steps taken. In other words, the total enthalpy change for a reaction is the same regardless of whether the reaction occurs in one step or several steps, as long as the initial and final states of the reactants and products are the same.
Mathematically, Hess's Law can be expressed as:
> ΔH° = ΣΔHn
Where ΔH° is the total enthalpy change, and ΣΔHn represents the sum of the enthalpy changes in each of the 'n' steps of the reaction.
The significance of Hess's Law lies in its application to determine the enthalpy changes in complex chemical reactions. This is achieved by breaking down the overall reaction into smaller, more manageable steps with known enthalpy values. These individual steps are then combined to calculate the enthalpy change for the overall reaction.
For example, consider the reaction:
> CS2(l) + 3 O2(g) → CO2(g) + 2 SO2(g)
To determine the enthalpy change (ΔH) for this reaction, we can use the following three reactions with known enthalpy values:
- C(s) + O2(g) → CO2(g); ΔHf = -393.5 kJ/mol
- S(s) + O2(g) → SO2(g); ΔHf = -296.8 kJ/mol
- C(s) + 2 S(s) → CS2(l); ΔHf = 87.9 kJ/mol
By manipulating these reactions and their corresponding enthalpy values, we can calculate the enthalpy change for the desired reaction.
Hess's Law is a valuable tool in determining the enthalpy changes in various chemical reactions, especially when direct measurement is challenging or unsafe. It highlights the concept of enthalpy as a state function, where the energy change depends only on the initial and final states of the system, regardless of the path taken.
Understanding FMLA: Father-in-Law Coverage Explained
You may want to see also
Frequently asked questions
Hess's Law, or Hess's Law of Constant Heat Summation, states that the total enthalpy change during a chemical reaction is independent of the sequence of steps taken. In other words, the overall enthalpy change is the same regardless of the route by which the chemical change occurs.
Hess's Law can be applied to entropy in the same way as it is applied to enthalpy. Entropy can be used in thermochemical equations, and the rules discussed in Thermochemical Equations 3, 4, and 5 (reversing signs when reversing equations, multiplying by constants) also apply to equations with entropy. This means that absolute entropies of substances can be used to determine entropy changes in new equations.
An example of Hess's Law applied to entropy is the calculation of the entropy change for the reaction between ethene and hydrogen chloride gases to make chloroethane gas. The standard enthalpy of formation values are used to complete the Hess's Law cycle, and the calculation is performed by writing down all the enthalpy changes that make up the two routes and equating them.
When applying Hess's Law to entropy, it is important to ensure that the reaction follows certain requirements. For example, if there are multiple steps, each equation must be correctly balanced, and all steps must start and end at constant temperatures and pressures to maintain constant reaction conditions. Additionally, the stoichiometric coefficients of products and reactants must be considered, as well as the absolute entropies for products and reactants.







