Raoult's law, named after French chemist François-Marie Raoult, states that the vapour pressure of a solution decreases when substances are mixed. Raoult's law is considered a law of thermodynamics and is used to calculate the relative lowering of vapour pressure of a solvent when a solute is added. The law assumes that the intermolecular forces between different molecules and similar molecules are equal. However, it is important to note that Raoult's law is only valid for ideal solutions, which are rare as they require chemical components to be chemically identical. In this context, the applicability of Raoult's law to immiscible solutions is a pertinent question.
| Characteristics | Values |
|---|---|
| Raoult's Law | States that a solvent's partial vapour pressure in a solution (or mixture) is equal to the vapour pressure of the pure solvent multiplied by its mole fraction in the solution |
| Raoult's Law Equation | Psolution = ΧsolventP0solvent |
| Ideal Solutions | Always completely miscible |
| Mixture of Immiscible Liquids | The vapour pressure of the solution increases and is equal to the vapour pressures of the two solvents independently |
| Boiling Point of Mixture | Remains the same as the pure solvent, but the temperature required to reach the boiling point decreases |
What You'll Learn

Raoult's Law and Partial Vapour Pressure
Raoult's law, established in 1887, is named after French chemist François-Marie Raoult. It is considered a law of thermodynamics.
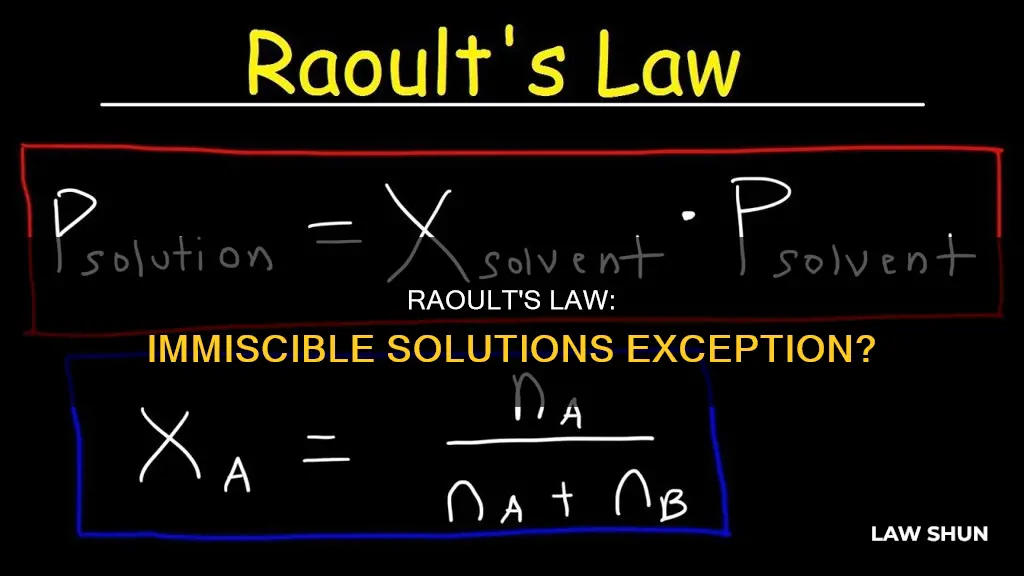
Raoult's law states that the vapour pressure of a solvent in a solution (or mixture) is equal to the vapour pressure of the pure solvent multiplied by its mole fraction in the solution.
Mathematically, Raoult's law can be written as:
Psolution = ΧsolventP0solvent
Where:
- Psolution = vapour pressure of the solution
- Χsolvent = mole fraction of the solvent
- P0solvent = vapour pressure of the pure solvent
Raoult's law can be applied to non-ideal solutions, but this requires considering the interactions between molecules of different substances. It is important to note that Raoult's law only works for ideal solutions. In reality, many liquids in mixtures do not have the same uniformity in terms of attractive forces, and these solutions tend to deviate from the law.
Raoult's law is used to calculate the relative lowering of vapour pressure when a solute is added to a volatile solvent mixture. In such cases, the vapour pressure of the solution decreases. However, in a mixture of immiscible liquids, the vapour pressure of the solution increases and is equal to the vapour pressures of the two solvents independently. Therefore, Raoult's law does not hold for mixtures of immiscible liquids.
The total vapour pressure above a solution depends on the vapour pressure of each component in its pure state and the mole fraction of each component in the solution. The vapour pressure of a liquid at a given temperature can be used to define its boiling point.
Exploring Legal Differences: English Law in Scotland
You may want to see also

Raoult's Law and Non-Volatile Solutes
Raoult's Law, established in 1887, is a law of thermodynamics named after French chemist François-Marie Raoult. It states that the vapour pressure of a solution (or mixture) is equal to the vapour pressure of the pure solvent multiplied by its mole fraction in the solution.
Mathematically, this can be written as:
Psolution = ΧsolventP0solvent
Where:
- Psolution is the vapour pressure of the solution
- Χsolvent is the mole fraction of the solvent
- P0solvent is the vapour pressure of the pure solvent
Raoult's Law applies to solutions in which the solute is non-volatile, meaning it has no tendency to form a vapour at the temperature of the solution. For example, a solution of salt in water.
The law explains that when a solute is added to a solvent, the vapour pressure of the solution decreases. This is because the solute molecules occupy the space between the solvent molecules on the surface of the solution, reducing the number of solvent molecules that can escape into the vapour phase.
Raoult's Law can be applied to non-ideal solutions by considering the interactions between molecules of different substances. However, it only works for ideal solutions, which are rare as they require that the solvent-solute interaction is the same as the solvent-solvent or solute-solute interaction.
The law also affects the boiling and freezing points of a solution. The boiling point of the solvent in a solution is higher than that of the pure solvent, while the freezing point is lower.
Idling Laws: Do They Apply to Semi Trucks?
You may want to see also

Raoult's Law and Volatile Solutions
Raoult's law, named after French chemist François-Marie Raoult, states that a solvent's partial vapour pressure in a solution is equal to the vapour pressure of the pure solvent multiplied by its mole fraction in the solution. In other words, it gives the relative lowering of vapour pressure of a dilute solution of a non-volatile solute.
Raoult's law is used to calculate the relative lowering of vapour pressure of a solvent when a solute is added. When a solute is added to a solvent, the vapour particles of the solute occupy the space between the solvent particles on the surface of the solution. This results in a lower vapour pressure of the solvent.
Raoult's law is applicable to volatile solutions. For example, consider a solution of volatile liquids A and B in a container. Since both A and B are volatile, there would be particles of both A and B in the vapour phase. The vapour particles of both liquids exert a partial pressure, contributing to the total pressure above the solution.
However, Raoult's law does not hold for a mixture of immiscible liquids. In such a mixture, the vapour pressure of the solution increases and is equal to the vapour pressures of the two solvents independently. Therefore, there is no lowering of the vapour pressure of the solution.
Leash Laws: Do They Apply to Dogs in Front Yards?
You may want to see also

Raoult's Law and Ideal Solutions
Raoult's law, proposed by French chemist François-Marie Raoult in 1887, is a relation of physical chemistry with implications in thermodynamics. The law states that the partial pressure of each component of an ideal mixture of liquids is equal to the vapour pressure of the pure component (liquid or solid) multiplied by its mole fraction in the mixture.
Raoult's law applies to ideal solutions, which are homogeneous solutions where the interaction between molecules of components (solute and solvents) is exactly the same as the interactions between the molecules of each component itself. These types of solutions follow Raoult's Law at almost all levels of concentration and temperatures.
An ideal solution can be obtained by mixing a solute and a solvent that consist of a similar molecular structure and size. For example, liquids A and B can be mixed, and the resulting solution will experience several intermolecular forces of attraction inside it: A-A intermolecular forces of attraction, B-B intermolecular forces of attraction, and A-B intermolecular forces of attraction. The solution is said to be an ideal solution when the intermolecular forces of attraction between A-A, B-B, and A-B are nearly equal.
Ideal solutions have the following characteristics:
- They follow Raoult's Law.
- The enthalpy of mixing of two components is zero, meaning no heat is released or absorbed during the mixing of two pure components to form an ideal solution.
- The volume of the mixing is equal to zero, meaning the total volume of the solution is exactly the same as the sum of the volumes of the solute and solution.
- The solute-solute interaction and solvent-solvent interaction are almost similar to the solute-solvent interaction.
Examples of ideal solutions include n-hexane and n-heptane, bromoethane and chloroethane, and ethyl bromide and ethyl iodide.
Raoult's law is also applicable to non-ideal solutions by incorporating factors that account for the interactions between molecules of different substances. However, ideal solutions are rare, as it is hard to find different chemical components that are chemically identical.
Congress and Slzndsr Laws: Who's Exempt?
You may want to see also

Raoult's Law and Intermolecular Forces
Raoult's law, established in 1887, is a principle of physical chemistry with applications in thermodynamics. It was proposed by French chemist François-Marie Raoult, who discovered that when substances were mixed in a solution, the vapour pressure of the solution decreased.
Raoult's law states that the partial vapour pressure of a solvent in a solution is equal to the vapour pressure of the pure solvent multiplied by its mole fraction in the solution. In other words, the vapour pressure of a solution is lowered when a solute is added.
Mathematically, Raoult's law can be written as:
Psolution = Χsolvent x P0solvent
Where:
- Psolution is the vapour pressure of the solution
- Χsolvent is the mole fraction of the solvent
- P0solvent is the vapour pressure of the pure solvent
Raoult's law assumes that intermolecular forces between different molecules are equal to those between similar molecules. It also assumes that the molar volumes of the components are the same. This is analogous to the ideal gas law, which assumes negligible intermolecular forces between molecules.
Raoult's law is useful for calculating the molecular mass of an unknown solute. It also helps determine the chemical potential of each component in a liquid mixture.
However, Raoult's law is only valid for ideal solutions, which are rare. In most cases, the interactions between molecules in a mixture are not uniform, leading to deviations from Raoult's law. These deviations can be negative or positive, depending on the relative strengths of intermolecular forces between like and unlike molecules.
In summary, Raoult's law describes the relationship between the vapour pressure of a solution and its components' mole fractions, assuming equal intermolecular forces and molar volumes. While it has practical applications, its validity is limited to ideal solutions.
Lemon Law and Trailers: What's the Verdict?
You may want to see also
Frequently asked questions
No, Raoult's law is only applicable to ideal solutions, which are rare. Immiscible solutions are not ideal solutions, as they consist of two separate phases that do not mix.
Raoult's law states that the partial vapour pressure of a solvent in a solution is equal to the vapour pressure of the pure solvent multiplied by its mole fraction in the solution. In other words, it describes the relationship between the vapour pressure of a solution and the concentration of its components.
Raoult's law is limited to ideal solutions, where the interactions between solvent-solute, solvent-solvent, and solute-solute are identical. Most solutions deviate from ideality due to differences in intermolecular forces, resulting in either negative or positive deviations from the expected vapour pressure.