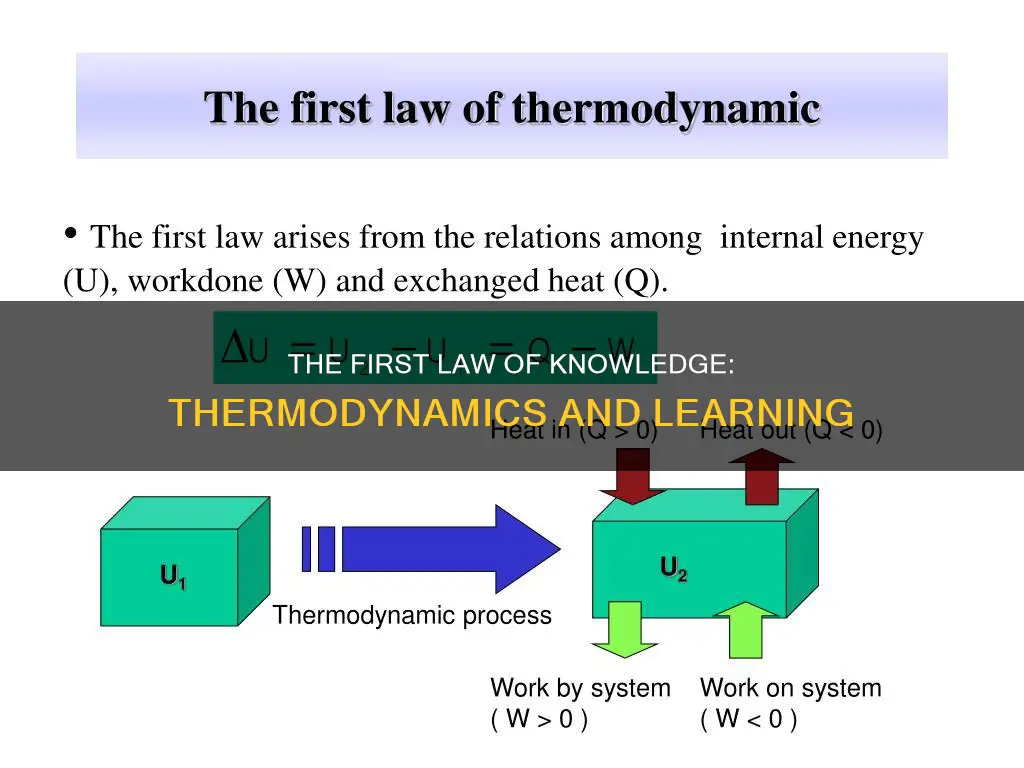
The first law of thermodynamics is a formulation of the law of conservation of energy, meaning that energy can be transferred or transformed from one form to another but cannot be created or destroyed. This law applies to thermodynamic processes and systems, and it distinguishes two principal forms of energy transfer: heat and thermodynamic work. The law also defines the internal energy of a system, which is a state variable like temperature or pressure.
The first law of thermodynamics can be applied to various fields and is not limited to closed systems. It is a fundamental principle in physics and other natural sciences. However, it does have certain limitations, such as not providing the direction of heat flow or explaining why heat cannot be spontaneously converted into work.
While the first law of thermodynamics provides a framework for understanding energy transformations, it does not address the feasibility of processes or changes of state. This is where the second law of thermodynamics comes into play, offering insights into the direction of heat flow and the spontaneity of processes.
| Characteristics | Values |
|---|---|
| First Law of Thermodynamics | Energy cannot be created or destroyed |
| First Law of Thermodynamics | Energy can be transferred between the system and the surroundings through the transfer of heat or by the performance of mechanical work |
| First Law of Thermodynamics | Energy can be transformed from one form to another |
| First Law of Thermodynamics | Energy can be converted to or from other forms of energy |
| First Law of Thermodynamics | Energy can be transferred from one location to another |
What You'll Learn
- The first law of thermodynamics is a version of the law of conservation of energy
- The law states that energy can be transferred between a system and its surroundings
- Energy can be converted from one form to another but cannot be created or destroyed
- The first law of thermodynamics is commonly called the conservation of energy
- The first law of thermodynamics is the least demanding to grasp

The first law of thermodynamics is a version of the law of conservation of energy
The first law of thermodynamics relates the various forms of kinetic and potential energy in a system to the work that a system can perform, and to the transfer of heat. It is sometimes taken as the definition of internal energy, and also introduces an additional state variable, enthalpy.
The first law of thermodynamics allows for many possible states of a system to exist. However, experience indicates that only certain states occur. This eventually leads to the second law of thermodynamics and the definition of another state variable called entropy.
The first law of thermodynamics is commonly called the conservation of energy. It is a general form of the law of conservation of energy that includes the effects of heat transfer and internal energy changes.
Abortion Laws: Ectopic Pregnancy Exclusion?
You may want to see also

The law states that energy can be transferred between a system and its surroundings
The first law of thermodynamics is a version of the law of conservation of energy, which states that energy cannot be created or destroyed. It can, however, be transferred from one form to another. The first law of thermodynamics relates to the changes in energy states due to work and heat transfer.
The first law of thermodynamics states that the energy of the universe remains the same. Though it may be exchanged between the system and the surroundings, it cannot be created or destroyed. This means that any gain in energy by the system will correspond to a loss in energy by the surroundings, and vice versa. The law defines the internal energy (E) as equal to the difference between the heat transfer (Q) into a system and the work (W) done by the system.
The first law is commonly called the conservation of energy. It is a subtle subject, and the first law is much more interesting than this remark might suggest. The law motivates the introduction and clarification of the meaning of the elusive concept of "energy". It allows for many possible states of a system to exist, but experience indicates that only certain states occur. This eventually leads to the second law of thermodynamics and the definition of another state variable called entropy.
The first law of thermodynamics is a formulation of the law of conservation of energy in the context of thermodynamic processes. It distinguishes two principal forms of energy transfer, heat and thermodynamic work, that modify a thermodynamic system containing a constant amount of matter. The law also defines the internal energy of a system, an extensive property for taking account of the balance of heat and work in the system.
The first law of thermodynamics is based on the law of conservation of energy, which states that energy cannot be created or destroyed, but it can be transferred from one form to another. The first law of thermodynamics is generally thought to be the least demanding to grasp, as it is an extension of the law of conservation of energy.
Lemon Law and Leases: What's the Verdict?
You may want to see also

Energy can be converted from one form to another but cannot be created or destroyed
The first law of thermodynamics, also known as the law of conservation of energy, states that energy cannot be created or destroyed. It can, however, be converted from one form to another. This means that the total energy of a system remains constant. For example, kinetic energy, the energy an object possesses when it moves, is converted to heat energy when a driver presses the brakes to slow down a car.
The first law of thermodynamics relates the various forms of kinetic and potential energy in a system to the work it can perform and the transfer of heat. It introduces the concept of internal energy, which is the energy associated with the molecules of the system, including kinetic and potential energy. The internal energy of a system increases or decreases depending on the work interaction that takes place across its boundaries. If work is done on the system, its internal energy increases, and if work is done by the system, its internal energy decreases.
The first law of thermodynamics allows for many possible states of a system to exist, but only certain states occur in nature. This is explained by the second law of thermodynamics, which states that in a natural thermodynamic process, the sum of the entropies of the interacting systems never decreases.
Conflict of Interest Laws: Who Are They For?
You may want to see also

The first law of thermodynamics is commonly called the conservation of energy
The first law of thermodynamics is commonly referred to as the conservation of energy. This is because the law states that energy cannot be created or destroyed, only transferred from one form to another. In other words, the total energy of a system remains constant.
The first law of thermodynamics is a formulation of the law of conservation of energy in the context of thermodynamic processes. It distinguishes two principal forms of energy transfer: heat and thermodynamic work. These two forms of energy transfer modify a thermodynamic system containing a constant amount of matter. The law also defines the internal energy of a system, which is an extensive property that accounts for the balance of heat and work in the system.
The first law of thermodynamics allows for many possible states of a system to exist, but only certain states are found to exist in nature. This observation is explained by the second law of thermodynamics.
The first law is a foundational principle in physics and is applicable beyond the field of thermodynamics, extending to other natural sciences.
Lemon Law and Mileage: What's the Verdict on 90K Miles?
You may want to see also

The first law of thermodynamics is the least demanding to grasp
The first law of thermodynamics is based on the law of conservation of energy, which states that energy cannot be created or destroyed, but can be transferred from one form to another. This is also referred to as the first law of thermodynamics for a closed system.
The first law of thermodynamics is generally considered the least demanding to understand as it is an extension of the law of conservation of energy. It is a simple concept: however much energy there was at the start of the universe, there will be that amount at the end.
The first law of thermodynamics states that the total energy of a system remains constant, even if it is converted from one form to another. For example, kinetic energy—the energy that an object possesses when it moves—is converted to heat energy when a driver presses the brakes on a car to slow it down.
The first law of thermodynamics relates the various forms of kinetic and potential energy in a system to the work that a system can perform, and to the transfer of heat. This law is sometimes taken as the definition of internal energy and also introduces an additional state variable, enthalpy.
The first law of thermodynamics allows for many possible states of a system to exist. However, experience indicates that only certain states occur. This eventually leads to the second law of thermodynamics and the definition of another state variable called entropy.
Castle Law: Business Application and Legal Boundaries
You may want to see also
Frequently asked questions
The first law of thermodynamics is a version of the law of conservation of energy, which states that energy cannot be created or destroyed, only transferred from one form to another.
The first law of thermodynamics can be applied to knowledge in the sense that knowledge cannot be created or destroyed, only transferred and converted from one form to another. For example, knowledge can be transferred from a book to a person or converted from written to verbal form.
While the first law of thermodynamics states that energy is conserved, it does not provide information about the direction of energy flow or explain why energy cannot be spontaneously converted into work. The second law of thermodynamics addresses these limitations by stating that heat cannot be spontaneously transferred from a cold body to a hot body.







