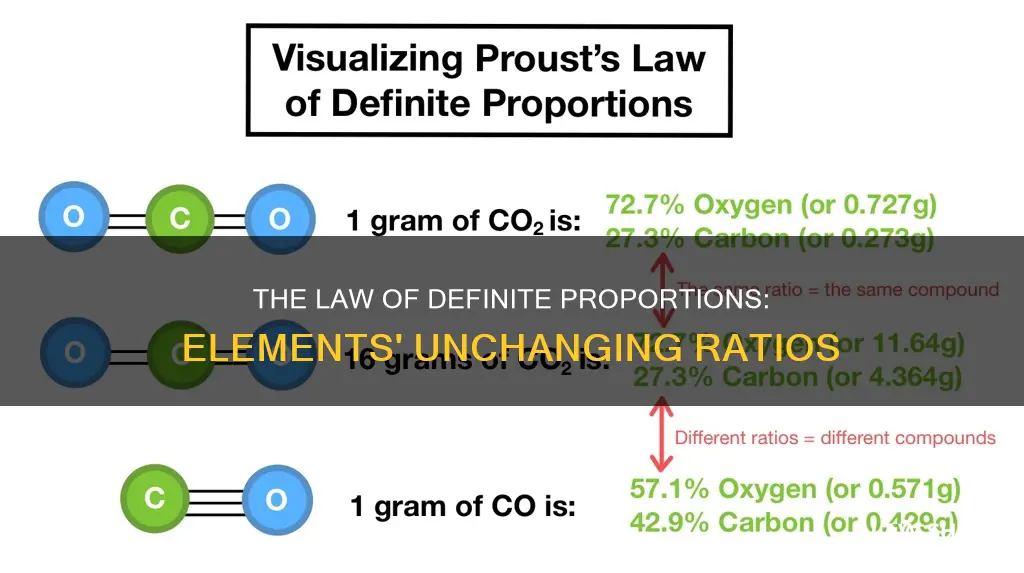
The law of definite proportions, also known as Proust's law or the law of constant composition, states that a given chemical compound always contains its component elements in a fixed ratio by mass. This means that the composition of a compound does not depend on its source or method of preparation. For example, any sample of pure water contains about 8/9 of oxygen by mass and 1/9 of hydrogen by mass. However, this law only applies to compounds, not individual elements.
| Characteristics | Values |
|---|---|
| Name | Law of Definite Proportions |
| Other Names | Proust's Law, Law of Constant Composition |
| Definition | States that a compound always contains exactly the same proportion of elements by mass |
| Application | Only applies to compounds, not elements |
| History | First proposed by Joseph Proust in 1794, but the first observation was made by Joseph Priestly and Antoine Lavoisier |
| Exceptions | Some compounds are non-stoichiometric and vary in elemental composition, e.g., wustite (iron oxide) |
What You'll Learn

The law applies only to compounds, not elements
The law of definite proportions, also known as Proust's Law or the law of constant composition, applies only to compounds and not to elements. This is because a compound is a substance formed when two or more elements are chemically bonded together, and an element contains only one type of atom.
The law of definite proportions states that a compound will always contain the same proportion of elements by mass, and this ratio is fixed regardless of the compound's source or method of preparation. For example, any sample of pure water will contain about 8/9 of its mass in oxygen and 1/9 in hydrogen. The law was formulated by French chemist Joseph-Louis Proust in 1797, although it was first proposed by English chemist and theologian Joseph Priestly and French chemist Antoine Lavoisier in 1794.
The law of definite proportions is fundamental to the study of stoichiometry in chemistry, but it is not universally true. There are exceptions, such as non-stoichiometric compounds whose elemental composition can vary from sample to sample. For instance, the iron oxide wüstite can contain between 0.83 and 0.95 iron atoms for every oxygen atom, resulting in a variable mass percentage of oxygen.
Additionally, the isotopic composition of an element can vary depending on its source, which can affect the mass of a pure stoichiometric compound. Polymers also vary in element composition by mass, although they are not considered true chemical compounds.
Foreign Investors: Navigating US Securities Laws
You may want to see also

The mass ratio of elements in a compound is fixed
The law of definite proportions, also known as Proust's law or the law of constant composition, states that a chemical compound always contains its component elements in a fixed ratio by mass. This means that the mass ratio of elements in a compound is always the same, regardless of the source of the elements or how the compound is prepared.
For example, in water, oxygen always makes up about 8/9 of the mass, while hydrogen makes up the remaining 1/9. This ratio is consistent across all samples of pure water, no matter their origin or how they were prepared. The law of definite proportions emphasizes that an atom of a specific element is identical to any other atom of that element. Therefore, an oxygen atom from silica is the same as an oxygen atom from the air.
The law of definite proportions was first proposed by French chemist Joseph Proust in 1794, although it was based on earlier work by English theologian and chemist Joseph Priestly and French chemist Antoine Lavoisier on the process of combustion. They observed that metals consistently combined with two proportions of oxygen. Proust's law was initially controversial, with some chemists, like his fellow Frenchman Claude Louis Berthollet, arguing that elements could combine in any proportion. However, it gained acceptance after English chemist John Dalton introduced his atomic theory in 1803, which explained that matter consisted of discrete atoms, with one type of atom per element, and that compounds were formed by combining different types of atoms in fixed proportions.
While the law of definite proportions is a fundamental concept in chemistry, it does have exceptions. Some compounds, known as non-stoichiometric compounds, can have varying elemental compositions from sample to sample. For instance, the iron oxide wüstite can have between 0.83 and 0.95 iron atoms for every oxygen atom, resulting in a mass composition ranging from 23% to 25% oxygen. Additionally, the isotopic composition of an element can vary depending on its source, leading to slight differences in the mass of a pure stoichiometric compound.
Lemon Law: Commercial Vehicle Rights and Protections
You may want to see also

The law is also known as Proust's Law
The law of definite proportions, also known as Proust's Law or the law of constant composition, was formulated by French chemist Joseph Proust in the Spanish city of Segovia in 1797. It states that a given chemical compound will always contain its component elements in a fixed ratio by mass, and this ratio will remain the same regardless of the compound's source or method of preparation.
Proust's Law can be understood as an extension of Lavoisier's Law of Conservation of Mass. It is different from the law of multiple proportions, although both are derived from Lavoisier's law. Proust's Law states that:
> A chemical compound always contains the same elements combined together in the same proportion by mass.
Proust's Law was formulated as a result of Proust's experiments with inorganic binary compounds, particularly iron oxides, which he first published in 1794. The law was controversial when it was first proposed, as it was opposed by other chemists, most notably Proust's fellow Frenchman Claude Louis Berthollet, who argued that elements could combine in any proportion. This debate demonstrated that, at the time, the distinction between pure chemical compounds and mixtures was not yet fully understood.
The law of definite proportions was later placed on a firm theoretical basis by John Dalton's atomic theory, which explained that matter consists of discrete atoms, with one type of atom for each element, and that compounds are made of combinations of different types of atoms in fixed proportions.
Leash Laws and Private Property: Understanding the Legal Boundaries
You may want to see also

The law forms the basis for the study of stoichiometry
The law of definite proportions, also known as Proust's Law or the law of constant composition, states that a given chemical compound always contains its component elements in a fixed ratio (by mass) and does not depend on its source or method of preparation. For example, in any sample of pure water, oxygen makes up about 8/9 of the mass, while hydrogen makes up the remaining 1/9.
The law was first proposed by French chemist Joseph Proust in 1797, although the observation was first made by English theologian and chemist Joseph Priestley, and French chemist Antoine Lavoisier, who studied combustion. Proust's law states that a compound will always contain exactly the same proportion of elements by mass. An element contains only one type of atom, so the law does not apply to it.
The law of definite proportions forms the basis for the study of stoichiometry. Stoichiometry is the study of the quantitative relationships that exist between the reactants and the products in chemical reactions. The law of definite proportions ensures that a chemical compound is always created using the same proportions, regardless of the amount of the compound being made. For example, the chemical compound for vinegar is always made from 2 atoms of carbon, 2 atoms of oxygen, and 4 atoms of hydrogen. There is no other combination of oxygen, carbon, and hydrogen that could be made to form vinegar.
Another example is water, which contains 88.81% oxygen and 11.19% hydrogen by mass. No matter how it was prepared or where the sample of water came from, the ratio of hydrogen to oxygen remains the same. This is a fundamental principle in stoichiometry, which allows us to understand the ratios of elements in compounds and the reactions that form them.
Insider Trading Laws: Exempting Congress?
You may want to see also

There are exceptions to the rule, such as non-stoichiometric compounds
The law of definite proportions, also known as Proust's law or the law of constant composition, states that a given chemical compound always contains its component elements in a fixed ratio (by mass). However, there are exceptions to this rule, including non-stoichiometric compounds.
Non-stoichiometric compounds are solid chemical compounds whose elemental composition cannot be expressed as a ratio of small whole numbers. In other words, the proportions of their constituent elements cannot be represented by a simple empirical formula. Instead, their formulas often include variables, such as "Fe0.95O", to indicate that the number of atoms of a particular element can vary. These compounds are typically inorganic solids and include various transition metal oxides, fluorides, hydrides, carbides, nitrides, sulfides, and tellurides.
The existence of non-stoichiometric compounds is related to defects in the lattice structures of crystalline substances. For example, in a sodium chloride crystal, if a sodium ion site is filled by a neutral sodium atom, the crystal defect is remedied, but the compound becomes non-stoichiometric because it now contains more sodium ions than chloride ions. Most non-stoichiometric compounds have compositions close to stoichiometric compounds, with only small deviations that can be detected through specialised analytical experiments.
Non-stoichiometric compounds exhibit unique electrical or chemical properties due to their defects. For instance, when atoms are missing, electrons can move through the solid more rapidly. These compounds have applications in ceramics, superconductors, and battery designs.
The classification of non-stoichiometric compounds can be based on the element in excess and how this excess occurs. For instance, oxygen-deficient or cation-vacancy types. They can also be categorised based on the magnitude of stoichiometric deviations, with narrow-range non-stoichiometric compounds deviating slightly from stoichiometric compositions, while wide-range non-stoichiometric compounds show a significant departure.
Lemon Law and Motorhomes: What's the Verdict?
You may want to see also