Several gas laws apply to altitude, including Boyle's Law, Charles's Law, Dalton's Law, Henry's Law, and Gay-Lussac's Law. These laws describe the behaviour of gases in relation to changes in volume, pressure, and temperature. For example, Boyle's Law states that the pressure of a gas is inversely proportional to its volume at a constant temperature, which means that as altitude increases, the volume of gas inside a closed space will expand due to decreased external pressure. Charles's Law describes the relationship between the volume of a gas and its temperature, while Dalton's Law explains how the total pressure of a gas mixture is determined by the pressures of its individual components. Henry's Law explains how the amount of a gas dissolved in a liquid is affected by the pressure of that gas, and Gay-Lussac's Law states that the pressure of a gas is related to its temperature. Understanding these gas laws is crucial for various applications, such as air travel and scuba diving, to ensure the safety and well-being of individuals exposed to changes in altitude.
| Characteristics | Values |
|---|---|
| Boyle's Law | The pressure of a gas is inversely proportional to its volume at a constant temperature. |
| Charles's Law | The volume of a gas is directly proportional to its temperature at a constant pressure. |
| Gay-Lussac's Law | The pressure of a gas is directly proportional to its temperature at a constant volume. |
| Dalton's Law | The total pressure of a gas mixture is equal to the sum of the partial pressures of its constituent gases. |
| Henry's Law | The amount of dissolved gas in a liquid is directly proportional to the partial pressure of that gas, at a constant temperature. |
| Fick's Law | Describes the diffusion of a gas across a membrane. |
What You'll Learn
- Boyle's Law: The inverse relationship between pressure and volume at a constant temperature
- Charles' Law: The relationship between volume and absolute temperature at constant pressure
- Gay-Lussac's Law: The relationship between pressure and absolute temperature at constant volume
- Avogadro's Law: The relationship between volume and the amount of gas at constant temperature and pressure
- Ideal Gas Law: The relationship between pressure, volume, number of moles, and absolute temperature

Boyle's Law: The inverse relationship between pressure and volume at a constant temperature
Boyle's Law, also known as the Boyle-Mariotte Law, is an empirical gas law that describes the relationship between the pressure and volume of a confined gas. The law was formulated by Anglo-Irish chemist Robert Boyle in 1662 and is based on the research of Richard Towneley and Henry Power.
Boyle's Law states that the pressure exerted by a given mass of an ideal gas is inversely proportional to the volume it occupies if the temperature and amount of gas remain unchanged within a closed system. This relationship can be expressed mathematically as:
$$P \propto \frac{1}{V}$$
Or:
$$PV = k$$
Where:
- $P$ is the pressure of the gas
- $V$ is the volume of the gas
- $k$ is a constant for a particular temperature and amount of gas
This means that as the volume of a gas increases, its pressure decreases proportionally, and vice versa, as long as the temperature remains constant. This relationship can also be expressed as:
$$P_1V_1 = P_2V_2$$
Where:
- $P_1$ and $V_1$ represent the initial pressure and volume
- $P_2$ and $V_2$ represent the final pressure and volume
This equation can be used to predict how changes in volume will affect the pressure exerted by a gas, as long as the temperature and amount of gas remain constant. For example, if you have a balloon filled with air, squeezing one end of the balloon will increase the pressure within, causing the un-squeezed section to expand outward. This is because the volume of the gas decreases as you squeeze the balloon, so the pressure must increase proportionally to maintain the constant product of $PV$.
Boyle's Law is particularly relevant when considering the effects of altitude on gases in closed cavities, such as in the human body. As altitude increases, ambient pressure decreases, and according to Boyle's Law, the volume of gas in enclosed spaces will expand. This can be observed in the expansion of a sealed bag of potato chips on a commercial flight or in the increase in volume of a pneumothorax during airlifting a patient to a hospital.
The Second Law's Scope: All Matter or Not?
You may want to see also

Charles' Law: The relationship between volume and absolute temperature at constant pressure
Charles's Law, also known as the Law of Volumes, describes the relationship between volume and absolute temperature at constant pressure. The law was named after scientist Jacques Charles, who formulated the original law in his unpublished work in the 1780s. It states that at constant pressure, the volume of a gas is directly proportional to its absolute temperature. This can be expressed as:
V ∝ T, or V/T = k, where k is a constant, and V1/T1 = V2/T2
Here, V is the volume of the gas, and T is the temperature of the gas measured in Kelvins. This law essentially describes how a gas expands as the temperature increases, and conversely, how a decrease in temperature leads to a decrease in volume.
For example, if you increase the volume of a gas and must keep the pressure constant, the only way to achieve this is for the temperature of the gas to increase as well. This relationship can be observed in a gas thermometer, where the change in volume of a gas is used to display the change in temperature.
Charles's Law can also be used to calculate the amount of nitrous oxide remaining in a gas cylinder. A nitrous oxide cylinder contains a mixture of gas and liquid at room temperature. As nitrous oxide is removed, the liquid boils, and the gas expands. By weighing the cylinder and applying Charles's Law, one can calculate the amount of nitrous oxide remaining.
In addition, Charles's Law can be used to understand the effects of altitude on gases in closed cavities within the body. As altitude increases, ambient pressure decreases, and according to Boyle's Law, volume expansion occurs in enclosed spaces. This can be observed in the expansion of a sealed bag of potato chips on a commercial flight.
Charles's Law, along with Boyle's Law, Gay-Lussac's Law, and Avogadro's Law, forms the basis for the Ideal Gas Law, which combines these individual gas laws into a single equation.
Limits and Laws: When One Limit Doesn't Exist
You may want to see also

Gay-Lussac's Law: The relationship between pressure and absolute temperature at constant volume
Gay-Lussac's Law, also known as the Third Gas Law or Amontons' Law, was formulated by French chemist Joseph Gay-Lussac in 1808. It states that the pressure exerted by a given mass of an ideal gas with a constant volume is directly proportional to its absolute temperature.
Mathematically, this can be expressed as:
P ∝ T
Or
P/T = K
Where P is the pressure, T is the absolute temperature, and K is a constant.
This law implies that the ratio of the initial pressure to temperature is equal to the ratio of the final pressure to temperature for a gas of a fixed mass kept at a constant volume. This can be derived from the pressure-temperature proportionality for gases, where P ∝ T for gases of fixed mass kept at constant volume.
Gay-Lussac's Law can be applied to understand the increase in pressure that accompanies an increase in the absolute temperature of a gas kept at a constant volume. For example, when a pressurised aerosol can is heated, the resulting increase in pressure exerted by the gases can lead to an explosion, which is why such containers often have warning labels. Another example is a pressure cooker, where heating increases the pressure exerted by the steam inside, leading to faster cooking.
Gay-Lussac's Law is one of the fundamental gas laws that, when combined mathematically, form the basis of the Ideal Gas Law. This law is a combination of Boyle's Law, Charles' Law, Gay-Lussac's Law, and Avogadro's Law.
Civil Rules in Florida Family Law Explained
You may want to see also

Avogadro's Law: The relationship between volume and the amount of gas at constant temperature and pressure
Avogadro's Law, also known as Avogadro's hypothesis or principle, is an experimental gas law that relates the volume of a gas to the amount of substance of gas present. The law was formulated by Amedeo Avogadro, who hypothesised in 1811 (or 1812, according to another source) that two samples of an ideal gas with the same volume and temperature and under the same pressure would contain the same number of molecules.
Avogadro's Law gives the relationship between volume and the amount of gas when pressure and temperature are held constant. The amount of gas is measured in moles, and the container holding the gas must be flexible so that it can expand or contract. If the amount of gas in a container is increased, the volume increases, and if the amount of gas is decreased, the volume decreases. This relationship is described by the following formula:
> {\displaystyle V\propto n}
>
> {\displaystyle {\frac {V}{n}}=k}
Where V is the volume of the gas, n is the amount of substance of the gas (measured in moles), and k is a constant for a given temperature and pressure.
The above equation can also be expressed as:
> {\displaystyle {\frac {V_{1}}{n_{1}}}={\frac {V_{2}}{n_{2}}}}
This shows that as the number of moles of gas increases, the volume of the gas also increases in proportion, and vice versa.
Avogadro's Law is a specific case of the ideal gas law, which combines several other gas laws, including Boyle's Law, Charles' Law, and Gay-Lussac's Law. The ideal gas law is expressed as:
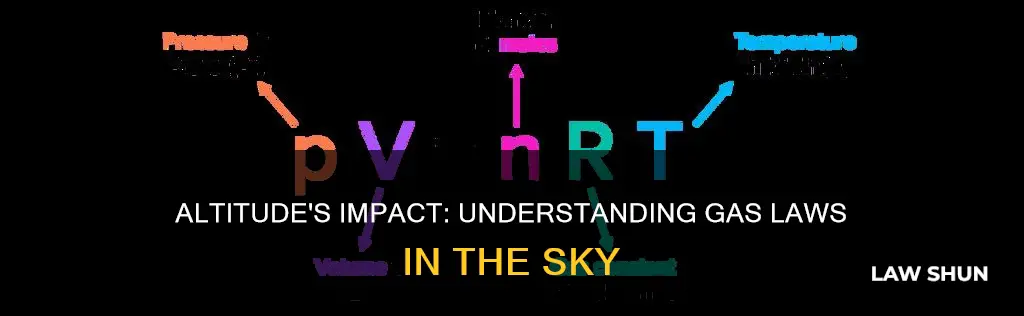
> {\displaystyle PV=nRT}
Where P is the pressure, V is volume, n is the number of moles, R is the universal gas constant, and T is the absolute temperature.
Whistle-blower Law: Contractor Rights and Protections
You may want to see also

Ideal Gas Law: The relationship between pressure, volume, number of moles, and absolute temperature
The Ideal Gas Law is a combination of Boyle's Law, Charles's Law, Gay-Lussac's Law, and Avogadro's Law. It states that the product of the pressure and volume of a gas is equal to the product of the number of moles of the gas, the ideal gas constant, and the absolute temperature.
Mathematically, this is expressed as:
PV = nRT
Where:
- P is the pressure of a gas
- V is the volume it occupies
- N is the number of moles of the gas
- R is the ideal gas constant
- T is its absolute temperature
The ideal gas constant, R, is equal to 8.314 J/(K·mol), or 0.820 (L·atm)/(K·mol). The constant is dependent on the units used for pressure, volume, and temperature.
The Ideal Gas Law can be used to calculate the unknown variable in the equation, as long as the other variables are known. For example, it can be used to calculate the unknown pressure, volume, number of moles, or absolute temperature of a gas.
The Ideal Gas Law is particularly useful for understanding the effects of altitude on gases in closed cavities within the body, such as in the field of aviation medicine. As altitude increases, ambient pressure decreases, and according to Boyle's Law, volume expansion occurs in enclosed spaces. This has implications for various medical conditions and equipment function, such as pneumothorax, bowel obstruction, and anti-shock trousers or MAST suits.
Additionally, the Ideal Gas Law can be applied to calculate the volume of oxygen available from a cylinder, which is relevant for patients requiring oxygen therapy or those undergoing hyperbaric therapy.
Boyles Law: Universal Gas Behavior Explained
You may want to see also
Frequently asked questions
Boyle's Law, which states that the pressure of a gas is inversely proportional to its volume at a constant temperature. This means that as altitude increases, and atmospheric pressure decreases, the volume of gas inside a closed space will expand.
Boyle's Law can cause issues such as Tension Pneumothorax, Pneumocephalus, and Barosinusitis. It is also important to consider when dealing with bodily injuries or diseases, such as pneumothorax or bowel obstruction.
Yes, Dalton's Law, Henry's Law, and Gay-Lussac's Law are also applicable. Dalton's Law states that the total pressure of a gas mixture is the sum of the partial pressures of its constituent gases. Henry's Law explains how the amount of gas dissolved in a liquid is affected by the pressure of that gas. Gay-Lussac's Law states that the pressure of a gas is related to its temperature.
The combination of these gas laws and their effects on pressure, volume, and temperature can lead to hypoxia, a condition where there is an inadequate supply of oxygen for proper cell function. This can be dangerous for anyone at high altitudes, especially those with existing respiratory or cardiovascular issues.







