The second law of thermodynamics is a physical law that states that the entropy of an isolated system will always increase over time. In other words, the disorder of a system will increase. This is because there is always a certain amount of energy in a system that is not available to do work, and this energy turns into heat, which increases the molecular disorder of the system.
The second law of thermodynamics is important because it allows us to predict how much heat a given machine will produce under different conditions. It also has applications in chemistry, cosmology, atmospheric sciences, biology, and many other fields.
| Characteristics | Values |
|---|---|
| Statement of the second law of thermodynamics | For a spontaneous process, the entropy of the universe increases. |
| For a spontaneous process, ΔSuniverse > 0. | |
| For a spontaneous process, ΔSsystem + ΔSsurroundings > 0 | |
| The entropy of an isolated system always increases over time | |
| The changes in the entropy in the universe can never be negative |
What You'll Learn

The second law of thermodynamics and the arrow of time
The second law of thermodynamics can be understood by considering a simple example. Imagine a cup of hot coffee left on a table. Initially, the coffee is hot and the table is cool. However, over time, the coffee will cool down and the table will warm up until they both reach the same temperature. This process is known as thermal equilibrium, and it is a result of the second law of thermodynamics. The heat from the coffee is transferred to the table, increasing the entropy of the system.
The second law of thermodynamics also has implications for our understanding of time. It suggests that time is not symmetric but has a preferred direction. This is known as the "arrow of time" and it points towards the direction of increasing entropy. This means that certain processes, like the cooling of coffee, are irreversible. Once the coffee has cooled down, it cannot spontaneously heat back up to its original temperature without an external source of energy.
The second law of thermodynamics also has implications for our understanding of the universe. It suggests that the universe is constantly moving towards a state of higher entropy. This could have implications for the future of the universe, such as the heat death hypothesis, which predicts that the universe will eventually reach a state of maximum entropy where no further change is possible.
The second law of thermodynamics also has practical applications. For example, it can be used to design more efficient heat engines and to understand the behaviour of complex systems, such as biological organisms. By considering the change in entropy, we can predict whether a process will occur spontaneously or not. This is particularly useful in chemistry and biology, where complex reactions and processes are taking place all the time.
Overall, the second law of thermodynamics and the arrow of time are deeply interconnected. The second law provides a fundamental understanding of the behaviour of matter and energy in the universe, and it has far-reaching implications for our understanding of time, the future of the universe, and the practical applications of thermodynamics in various fields.
The Dark History of Jim Crow Laws and Their Reach
You may want to see also

The second law of thermodynamics and the age of the Earth
The second law of thermodynamics states that the entropy of the entire universe, as an isolated system, will always increase over time. It also states that the changes in the entropy of the universe can never be negative. This is often referred to as the "arrow of time", suggesting that time itself is asymmetric with respect to the order of an isolated system.
The second law was first formulated in the 19th century by the Scottish physicist William Thomson (Lord Kelvin) and the German physicist Rudolf Clausius. The law describes the amount of work that can result from a transfer of heat. It is a physical law based on universal empirical observation concerning heat and energy interconversions.
The second law of thermodynamics played a role in determining the age of the Earth. In the 1800s, scientists were trying to determine the age of the planet and understand how it transformed. Lord Kelvin hypothesised that the Earth's surface was once extremely hot and that it was cooling slowly. Using thermodynamics, he concluded that the Earth was at least 20 million years old, as it would take that long for the planet to cool to its current state. This estimate was inaccurate, as scientists at the time were not aware of radioactivity. However, Kelvin's use of the second law allowed him to predict a more accurate age than other scientists at the time.
The second law of thermodynamics also has implications for biology and evolution. It posits that the transfer of energy involves some heat being released. This inefficient energy transfer plays a role in many biological systems and food chains. Evolution does not violate the second law, as it only applies to systems with no external energy sources, and the Earth receives energy from the Sun.
RV Lemon Law: What's the Deal?
You may want to see also

The second law of thermodynamics and evolution
The second law of thermodynamics states that the entropy of the entire universe as an isolated system will always increase over time. The law also states that the changes in the entropy in the universe can never be negative. The second law applies to the universe as a whole, but it can also be applied to subsystems of the universe.
The second law of thermodynamics is often used as an argument against the theory of evolution. Critics claim that the second law contradicts the theory of evolution because, as per the law, disorder or entropy always increases or remains the same over time. However, this objection is based on a misunderstanding of the second law. The law states that any isolated system will increase its total entropy over time. An isolated system is defined as one without any outside energy input. The Earth is not an isolated system as it receives constant energy input from the Sun. Therefore, the second law does not apply to the Earth and evolution.
Evolution can be viewed as an open system, where matter and energy are exchanged with the surroundings. Ilya Prigogine, a physical chemist, wrote about dissipative structures as a mechanism that could bring about self-organization. Dissipative structures refer to structures that can arise when an environment is far from thermodynamic equilibrium. Living organisms are highly ordered systems that are far from thermodynamic equilibrium. They maintain their low entropy by taking in free energy (nutrients) and expelling entropy (heat and waste).
The second law of thermodynamics can be stated as: "For a spontaneous process, the entropy of the universe increases." This means that for any spontaneous process, the entropy, which is related to randomness or disorder, of the universe increases. The second law does not mean that the entropy of a reaction must be positive. If the entropy of a reaction is negative, then the second law states that the entropy of the surroundings must be positive and greater in magnitude so that the total entropy of the universe is positive.
The second law of thermodynamics is one of the most misunderstood aspects of physics. However, it is important to understand its implications for evolution and the development of complex life.
Romeo and Juliet Laws: Do They Protect Nude Minors?
You may want to see also

The second law of thermodynamics and the Carnot cycle
The second law of thermodynamics states that the entropy of the entire universe, as an isolated system, will always increase over time. It also states that the changes in the entropy in the universe can never be negative. The law is based on universal empirical observation concerning heat and energy interconversions.
The law was formulated by French scientist Nicolas Léonard Sadi Carnot in 1824, who is considered the "father of thermodynamics". The law, in one of its forms, states that heat transfer occurs spontaneously from higher- to lower-temperature bodies but never spontaneously in the reverse direction. This is the basis of the Carnot cycle, which is the most efficient cyclical process possible and uses only reversible processes through its cycle.
The Carnot cycle is a theoretical cycle that was developed by Sadi Carnot. It is based on the theoretical analysis of the flow of heat in steam engines. The centerpiece of that analysis, now known as a Carnot engine, is an ideal heat engine that is operated in the limiting mode of extreme slowness, known as quasi-static. This means that the heat and work transfers are between subsystems that are always in their own internal states of thermodynamic equilibrium.
The Carnot cycle comprises two isothermal and two adiabatic processes. Both isothermal and adiabatic processes are, in principle, reversible. The efficiency of a quasi-static or reversible Carnot cycle depends only on the temperatures of the two heat reservoirs and is the same, whatever the working substance. A Carnot engine operated in this way is the most efficient possible heat engine using those two temperatures.
The second law of thermodynamics can be restated in terms of the Carnot cycle, and so what Carnot actually discovered was this fundamental law. Any heat engine employing the Carnot cycle is called a Carnot engine.
Mendel's Law of Inheritance and Corn: A Study
You may want to see also

The second law of thermodynamics and entropy
The second law of thermodynamics is a physical law that applies to all matter and is based on universal empirical observation concerning heat and energy interconversions. It establishes the concept of entropy as a physical property of a thermodynamic system.
The second law of thermodynamics can be stated in three synonymous ways:
- For a spontaneous process, the entropy of the universe increases.
- For a spontaneous process, ΔSuniverse > 0.
- For a spontaneous process, ΔSsystem + ΔSsurroundings > 0
The last statement of the second law of thermodynamics divides the universe into two parts: the system (what you're investigating) and the surroundings (everything in the universe besides the system). The second law does not mean that ΔSreaction must be positive, as ΔSreaction is just the ΔSsystem, which can be either positive or negative. But if ΔSreaction for a spontaneous reaction is negative, then the second law does mean that ΔSsurroundings must be positive and of greater magnitude so that ΔSsystem + ΔSsurroundings > 0.
The second law of thermodynamics affirms that the entropy of an isolated system always increases over time. The second law also states that the changes in the entropy in the universe can never be negative. The second law can also be stated as "all spontaneous processes produce an increase in the entropy of the universe".
The second law of thermodynamics was formulated by Rudolf Clausius in the 1850s and included his statement that heat can never pass from a colder to a warmer body without some other change occurring at the same time. The second law was also formulated by William Thompson (Lord Kelvin) and Constantin Carathéodory.
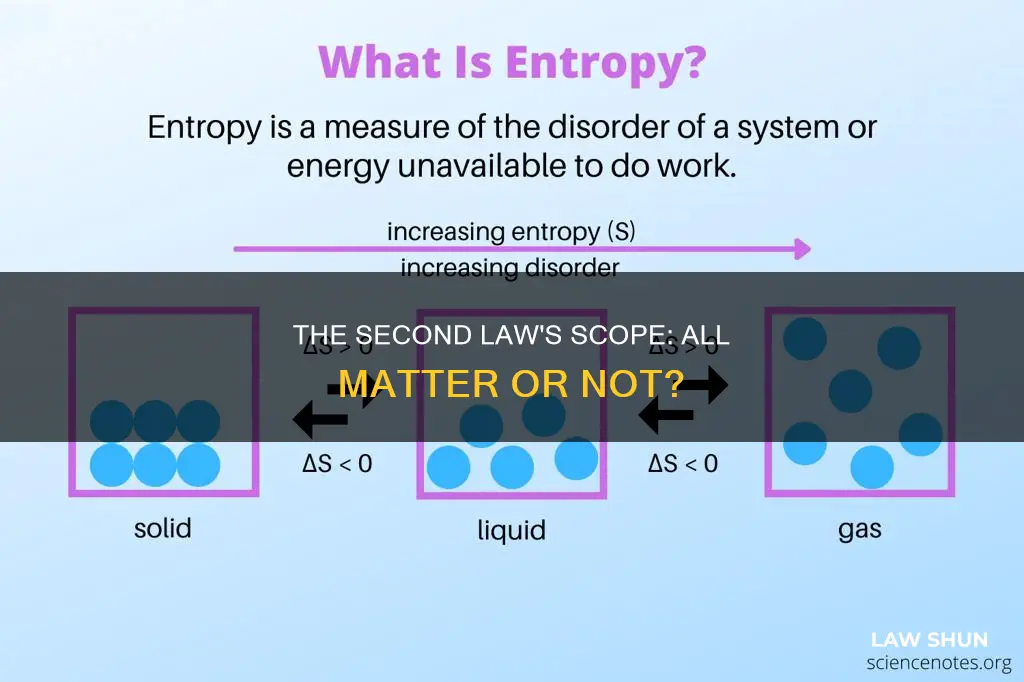
Entropy is a physical property that measures the amount of thermal energy in a system that is unavailable for doing useful work. The energy that can’t do work turns into heat, and the heat increases the molecular disorder of the system. Entropy can also be thought of as a measurement of that disorder. The increasing entropy (ΔS) equates to the heat transfer (ΔQ) divided by the temperature (T). This is why the second law of thermodynamics can be expressed with the formula ΔS =ΔQ / T.
Thermodynamics in Space: Do the Laws Apply?
You may want to see also
Frequently asked questions
Yes, the second law of thermodynamics applies to the universe. The law states that the entropy of an isolated system, such as the universe, will always increase over time.
The second law of thermodynamics is a fundamental law of nature that covers numerous phenomena and has deep practical and philosophical consequences. It is concerned with the direction of natural processes and asserts that a natural process runs only in one sense and is not reversible.
The second law of thermodynamics can be expressed mathematically as ΔS =ΔQ / T, where ΔS is the change in entropy, ΔQ is the heat transfer, and T is the temperature.