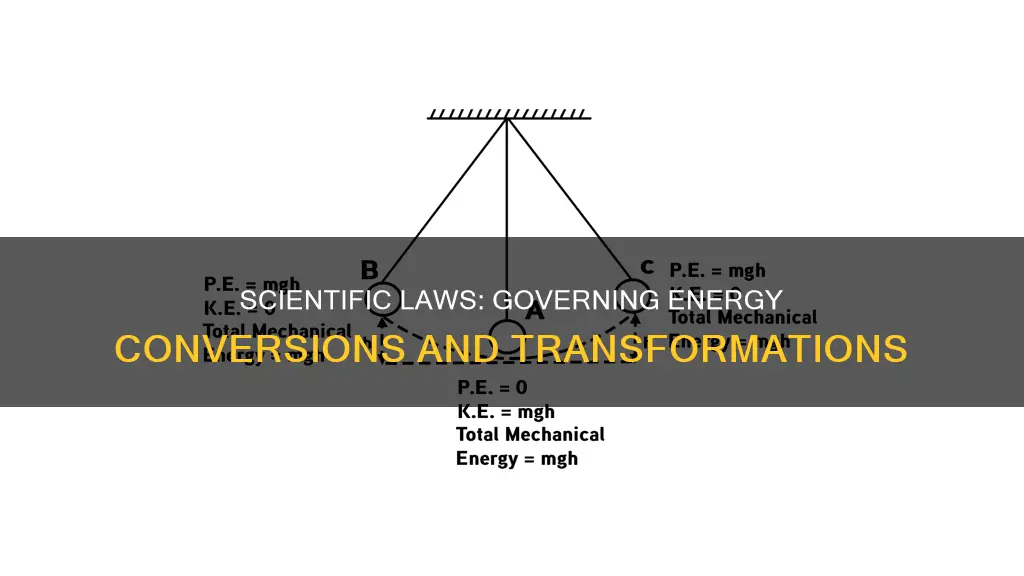
Energy conversion, also known as energy transformation, is the process of changing one form of energy into another. The law of conservation of energy states that energy cannot be created or destroyed, only transformed from one form to another. This law is a fundamental principle in physics and is based on the idea that the total amount of energy in a closed system remains constant over time. This means that the total energy within a system can only change if energy enters or leaves the system. This law has important implications for various scientific fields, including thermodynamics, and has guided the development of energy-conversion technologies.
| Characteristics | Values |
|---|---|
| Definition of Energy Conversion | The process of changing one form of energy into another |
| Law of Energy Conversion | The law of conservation of energy states that energy can neither be created nor destroyed, only converted from one form to another |
| Forms of Energy | Thermal energy, electrical energy, nuclear energy, electromagnetic energy, mechanical energy, chemical energy, sound energy, etc. |
| Energy Transformation | The term used when energy changes forms from one to another |
| Energy Transfer | The movement of energy from one location to another |
| Efficiency | The amount of useful energy obtained from a system |
| Examples of Energy Conversion | Rubbing hands together for warmth (kinetic energy to thermal energy); a falling object speeding up (gravitational potential energy to kinetic energy); using a battery-powered torchlight (chemical to electrical energy) |
What You'll Learn

The first law of thermodynamics
> The change in internal energy of a system equals the net heat transfer into the system minus the net work done by the system.
Mathematically, this is represented as:
> ΔU = Q − W
Here, ΔU is the change in internal energy U of the system; Q is the net heat transferred into the system (the sum of all heat transfers into and out of the system); and W is the net work done by the system (the sum of all work done on or by the system).
Scooters and the 7500-Mile Law: What's the Verdict?
You may want to see also

Energy conversion in automobiles
Energy conversion, also known as energy transformation, is the process of changing one form of energy into another. This process occurs constantly, and there are many forms of energy, including thermal, electrical, nuclear, electromagnetic, mechanical, chemical, and sound energy. The law of conservation of energy, also known as the first law of thermodynamics, states that energy can neither be created nor destroyed, only transformed from one form to another.
In automobiles, energy conversion occurs through a series of transformations. Firstly, chemical energy in the fuel is converted into kinetic energy of the expanded gas through combustion. This kinetic energy of the expanding gas is then converted into linear piston movement. The linear piston movement is then converted into rotary crankshaft movement, and finally, the rotary movement of the drive wheels is converted into the linear motion of the automobile.
In this process, the chemical energy in the fuel is transformed into mechanical energy to power the car's movement. This involves an intermediate step where the chemical energy is first converted into thermal energy, which is then converted into mechanical energy.
The conversion of energy in automobiles is an example of a complex energy conversion system, where energy undergoes multiple transformations through various intermediate forms. These systems are subject to limitations as dictated by the laws of thermodynamics and other scientific principles.
English Law in the US: Who Rules?
You may want to see also

Energy conversion in power plants
Energy conversion is the process of changing one form of energy into another. The law of conservation of energy states that energy cannot be created or destroyed, only transformed from one form to another. This is also known as the first law of thermodynamics.
Power plants are a prime example of energy conversion technology, taking fuels such as coal, natural gas, or uranium and converting them into electricity. This process involves several steps, each involving a different form of energy.
In a coal-fired power plant, the chemical energy in the coal is first converted into thermal energy through combustion. The thermal energy of the exhaust gases is then used to heat water and produce steam. This steam travels through pipes at high pressures and speeds, eventually expanding through a turbine, converting thermal energy into mechanical energy. Finally, the motion of the turbine spins an electrical generator, converting mechanical energy into electrical energy.
Nuclear power plants follow a similar process, converting nuclear fuels into thermal and then electrical energy. Other energy conversion technologies include cars, batteries, heaters, and generators.
The efficiency of energy conversion systems is dictated by the laws of thermodynamics and other scientific principles. Direct energy conversion systems, such as solar cells and fuel cells, bypass the intermediate step of converting energy into heat before turning it into electricity.
Boyles Law: Universal Gas Behavior Explained
You may want to see also

Mass-energy equivalence
> E=mc^2
In this formula, E is the energy of a particle, m is its mass, and c^2 is the speed of light squared. This formula implies that a small amount of "rest mass" (the mass of a stationary object) corresponds to a large amount of energy.
The mass-energy equivalence principle states that all objects with mass have a corresponding intrinsic energy, even when they are stationary. This is because the energy of an object is the product of its mass and the speed of light squared. As the speed of light is a very large number, this results in a large amount of energy.
The principle of mass-energy equivalence has significant implications for various fields of physics, including nuclear and particle physics. For example, it explains why the mass of the atoms that come out of a nuclear reaction is less than the mass of the atoms that went in, with the difference showing up as heat and light. It also has applications in the study of thermodynamics and the conversion of energy from one form to another.
The concept of mass-energy equivalence arose from special relativity and was first proposed by Einstein in 1905. It states that mass and energy are two ways of measuring what is essentially the same underlying quantity. This means that mass can be converted into energy and vice versa, as long as the total amount of mass-energy remains the same.
Fourier's Law: Understanding Its Role in Convection
You may want to see also

Limitations of thermal energy conversion
Energy conversion is the process of changing one form of energy into another. Energy exists in various forms, including thermal energy, electrical energy, nuclear energy, electromagnetic energy, mechanical energy, chemical energy, and sound energy. The law of conservation of energy states that energy cannot be created or destroyed, only transformed or transferred from one form to another.
Now, let's discuss the limitations of thermal energy conversion in 4-6 paragraphs:
Thermal energy conversion has some inherent limitations that are important to understand. Firstly, thermal energy, unlike other forms of energy, often cannot be converted into other forms of energy. This is because thermal energy represents a highly disordered state, with energy spread randomly among the microscopic particles in a system. The measure of this disorder is entropy, and according to the second law of thermodynamics, the entropy of an isolated system never decreases. Therefore, converting thermal energy into a more ordered, useful form of energy is challenging.
The efficiency of converting thermal energy into other forms is typically much less than 100%. This is due to the nature of thermal energy, which is dispersed among many available states. To increase the efficiency of energy transformation, it is often desirable to avoid thermal conversion and instead focus on direct conversions between other forms of energy. For example, in nuclear reactors, the initial conversion of kinetic energy to thermal energy and then to electrical energy results in lower efficiency than if the kinetic energy were directly converted to electrical energy.
Another limitation of thermal energy conversion is the need to transfer excess heat to a thermal reservoir at a lower temperature. This is necessary to maintain the overall increase in entropy required by the second law of thermodynamics. As a result, only a portion of thermal energy can be converted into other useful forms of energy, while the rest must be dissipated as waste heat. This further reduces the efficiency of thermal energy conversion processes.
Furthermore, the conversion of thermal energy to other forms is often subject to fundamental limitations imposed by the laws of thermodynamics and other scientific principles. These limitations restrict the maximum achievable efficiency and require careful engineering to optimize energy transformation processes.
In summary, while thermal energy conversion plays a crucial role in many energy systems, it has limitations due to the nature of thermal energy as a highly disordered state. The low efficiency of conversion, the need to dissipate waste heat, and the constraints imposed by thermodynamics make it challenging to fully utilize thermal energy for useful work. Overcoming these limitations involves exploring direct energy conversion methods and improving the understanding of the underlying scientific principles.
Police and HIPAA: Understanding Legal Boundaries and Applicability
You may want to see also
Frequently asked questions
The law of conservation of energy states that energy can neither be created nor destroyed. It can only be converted from one form to another. The total amount of energy in a system remains constant unless energy is added from an external source.
In a car engine, chemical energy in the gasoline is converted into mechanical energy. This is an example of energy transformation, where one form of energy is changed into another.
A microphone converts sound energy into electrical energy. This process demonstrates the law of conservation of energy, as the sound is transformed without being created or destroyed.







