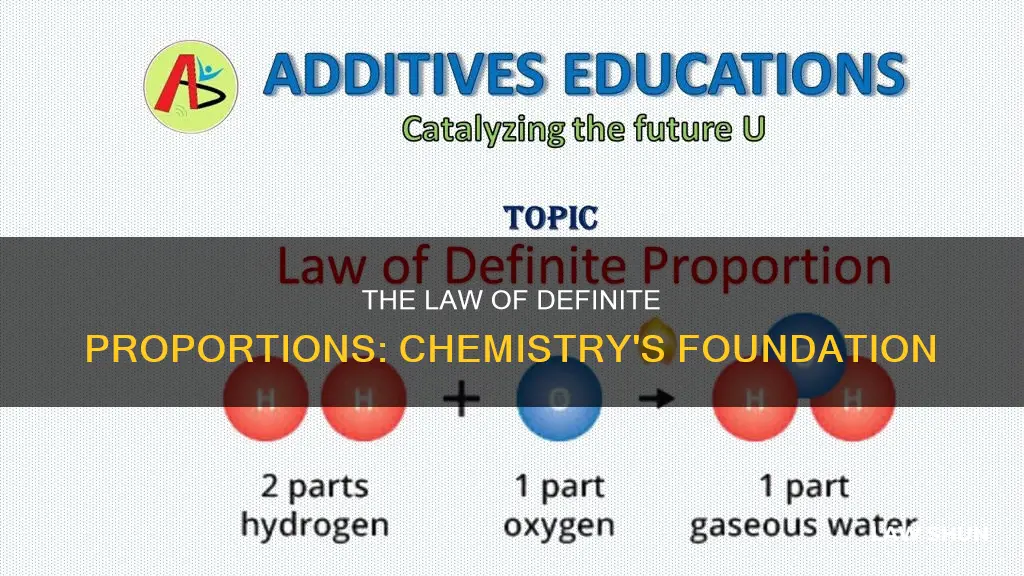
The law of definite proportions, also known as Proust's Law or the law of constant composition, is a fundamental concept in chemistry. It states that a chemical compound will always contain its constituent elements in a fixed ratio by mass, regardless of its source or method of preparation. This principle, first formulated by Joseph Proust in 1797, forms the basis of stoichiometry, alongside the law of multiple proportions.
| Characteristics | Values |
|---|---|
| Name | Law of Definite Proportions, Proust's Law, Law of Constant Composition |
| Definition | Every definite compound always contains the same elements in the same proportions by weight or mass |
| Discovery | French chemist Joseph Proust (1754-1826) |
| First Publication | Joseph Priestly and Antoine Lavoisier in 1794 |
| Basis For | Stoichiometry |
| Exceptions | Non-stoichiometric compounds, isotopes, polymers |
What You'll Learn

The law of constant composition
For example, in any sample of pure water, oxygen makes up about 8/9 of the mass, while hydrogen accounts for the remaining 1/9. This ratio is consistent across different samples of water, regardless of their origin or how they were obtained. The law of constant composition extends beyond water, applying to all chemical compounds. For instance, in table salt, the atomic weight of sodium is approximately 23, and that of chlorine is about 35. Therefore, according to the law, dissociating 58 grams of NaCl would yield around 23 grams of sodium and 35 grams of chlorine.
While the law of constant composition is a cornerstone of chemistry, it does have exceptions. Some compounds, known as non-stoichiometric compounds, exhibit variations in their elemental composition across different samples. An example is wüstite, an iron oxide with an iron-to-oxygen ratio ranging from 0.83 to 0.95, resulting in an oxygen content of 23% to 25% by mass. The ideal formula for iron oxide is FeO, but the crystal structure of wüstite (Fe0.95O) introduces variations. Additionally, the isotopic composition of an element can vary depending on its source, leading to slight differences in the mass of a pure stoichiometric compound.
Sunlight and Earth: Inverse Square Law's Applicability
You may want to see also

Stoichiometry
- Determine the amount of products and reactants that are produced or needed in a given reaction.
- Convert from grams to moles using molar mass as the conversion factor.
- Balance chemical equations.
- Find the right amount of one reactant to "completely" react with the other reactant in a chemical reaction.
- Find the limiting reagent and the percent yield.
The term stoichiometry was first used by Jeremias Benjamin Richter in 1792, and is derived from the Ancient Greek words "stoikhein", meaning element, and "metron", meaning measure.
Trans Law Locker Room Conundrum: Inclusion or Invasion?
You may want to see also

Atomic theory
The law of definite proportions, also known as Proust's law or the law of constant composition, is a fundamental concept in chemistry. It states that a given chemical compound consistently maintains a fixed ratio of its constituent elements by mass, regardless of its source or method of preparation. This principle was first introduced by French chemist Joseph-Louis Proust in 1797, marking a significant contribution to the field of chemistry.
This law can be explained by considering the example of water. According to the law of definite proportions, water always consists of 8/9 oxygen by mass and 1/9 hydrogen by mass. This ratio remains constant, irrespective of the source or method of preparation of the water sample. For instance, whether the oxygen comes from silica or the oxygen in the air, an atom of oxygen is identical to any other atom of oxygen.
The law of definite proportions played a crucial role in the development of atomic theory by John Dalton, commencing in 1805. Dalton's atomic theory provided a comprehensive explanation of matter, proposing that matter is composed of discrete atoms, with each element characterised by a unique type of atom. Furthermore, his theory asserted that chemical compounds are formed by combining different types of atoms in fixed proportions. This theory successfully reconciled the law of definite proportions with the understanding of atomic structure, paving the way for significant advancements in chemistry.
It is worth noting that while the law of definite proportions serves as a cornerstone of modern chemistry, it does have certain exceptions. Some compounds, known as non-stoichiometric compounds, exhibit variations in their elemental composition across different samples. Wüstite, an iron oxide, is one such example. Its formula is typically written as Fe0.95O due to variations in its crystal structure, resulting in iron oxide ratios ranging from 0.83 to 0.95 iron atoms for every oxygen atom.
In summary, the law of definite proportions holds significant importance in chemistry, providing a foundational understanding of the fixed elemental ratios in chemical compounds. However, it is essential to acknowledge the existence of exceptions to this law, such as non-stoichiometric compounds, which exhibit variability in their elemental composition.
Applying Coulomb's Law to Ions: A Practical Guide
You may want to see also

Non-stoichiometric compounds
The existence of non-stoichiometric compounds is related to the presence of defects in the lattice structures of crystalline substances. For example, a sodium chloride crystal that lacks a sodium ion and a chloride ion is still stoichiometric because the numbers of sodium and chloride ions are the same. However, if the sodium ion site is filled by a neutral sodium atom, which gives up its valence electron to fill the chloride ion site, the crystal defect is remedied, but the compound is now non-stoichiometric because it contains more sodium ions than chloride ions.
Most non-stoichiometric compounds have compositions that are close to those of stoichiometric compounds. They can be classified into anion vacancy types, cation vacancy types, anion interstitialcy types, and cation interstitialcy types. An example of a cation vacancy type is Fe1−xO, where there is a deviation from the ideal stoichiometric composition of FeO due to the absence of iron ions.
Healthcare and American Law: Who's in Charge?
You may want to see also

Isotopes
The law of definite proportions, also known as Proust's law or the law of constant composition, states that a given chemical compound always contains its component elements in a fixed ratio (by mass) and does not depend on its source or method of preparation. For example, oxygen makes up about 8/9 of the mass of any sample of pure water, while hydrogen makes up the remaining 1/9 of the mass.
For example, let's consider water (H2O). The law of definite proportions tells us that, in any sample of water, the mass ratio of hydrogen to oxygen is fixed at 1:8. However, the specific isotopes of hydrogen and oxygen present in the water can vary. Hydrogen has three common isotopes: protium (1 proton, 0 neutrons), deuterium (1 proton, 1 neutron), and tritium (1 proton, 2 neutrons). Oxygen has three stable isotopes: 16O (8 protons, 8 neutrons), 17O (8 protons, 9 neutrons), and 18O (8 protons, 10 neutrons). So, while the ratio of hydrogen to oxygen atoms in water is always 2:1, the specific isotopes of hydrogen and oxygen can vary, leading to slight variations in the overall mass of the water molecule.
This variation in isotopic composition is measurable with modern instrumentation and has important applications in radiometric dating. By analyzing the relative abundances of certain isotopes in a sample, scientists can determine the age of materials, such as rocks, fossils, and archaeological artifacts. This technique is based on the principle that certain isotopes decay over time at known rates, allowing for the calculation of the age of the sample.
In summary, while the law of definite proportions states that a given chemical compound will always have its elements in a fixed ratio by mass, the specific isotopes present can vary depending on the source of the compound. This variation in isotopic composition has important applications in fields such as radiometric dating and contributes to our understanding of the natural world.
Congress and Slzndsr Laws: Who's Exempt?
You may want to see also
Frequently asked questions
The Law of Definite Proportions, also known as Proust's Law or the Law of Constant Composition, is a fundamental principle in chemistry. It states that a given chemical compound will always contain its constituent elements in a fixed ratio by mass, regardless of its source or method of preparation.
One example is water. No matter where the sample of water is from, it will always contain about 8/9 of its mass as oxygen and the remaining 1/9 as hydrogen. Another example is carbon dioxide, which always consists of one atom of carbon and two atoms of oxygen.
The French chemist Joseph-Louis Proust is credited with the discovery of the law in 1797. However, it was first published as a scientific proposal in 1794 by Joseph Priestly and Antoine Lavoisier, who noted that metals always combine with two proportions of oxygen.