The second law of thermodynamics states that the entropy of the universe, as an isolated system, will always increase over time. In other words, the law explains that the disorder within a system will increase as time passes. This is often referred to as the arrow of time, a concept that applies to every area of science.
The second law was first formulated by Nicolas Léonard Sadi Carnot, a French physicist, in 1824. However, the current form of the law uses entropy instead of caloric, which was the term used by Sadi Carnot to describe the law. The law was further developed by Rudolf Clausius, a German physicist, who created the Clausius statement, which says that heat generally cannot flow spontaneously from a material at a lower temperature to a material at a higher temperature.
What You'll Learn

The second law of thermodynamics and the arrow of time
The second law of thermodynamics states that the entropy of the universe, as an isolated system, will always increase over time. The law also states that the changes in entropy in the universe can never be negative. The second law of thermodynamics is about the nature of energy and states that there is a natural tendency of any isolated system to degenerate into a more disordered state. The law indicates the irreversibility of natural processes and, in many cases, the tendency of natural processes to lead towards spatial homogeneity of matter and energy, especially of temperature.
The second law of thermodynamics gives us the thermodynamic arrow of time. The law states that the total entropy of a system either increases or remains constant in any spontaneous process; it never decreases. This implies that the direction of time always flows in the direction of increasing entropy.
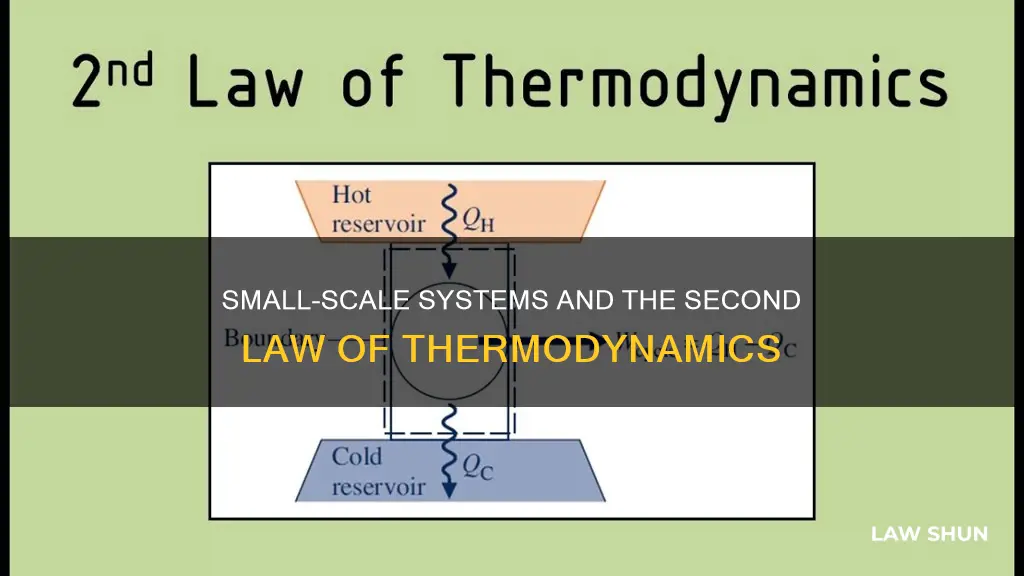
The second law of thermodynamics can be applied to small things. For example, the law can be applied to the expansion of a puff of gas introduced into one corner of a vacuum chamber. The gas expands to fill the chamber, but it never regroups on its own in the corner. The second law of thermodynamics states that heat transfers energy spontaneously from higher- to lower-temperature objects, but never spontaneously in the reverse direction. This is because entropy increases for heat transfer of energy from hot to cold.
HIPAA Laws and the President: Who's Exempt?
You may want to see also

The law's implications for the fate of the universe
The second law of thermodynamics has profound implications for the fate of the universe. This law, based on empirical observation, states that heat flows spontaneously from hotter to colder regions of matter and that not all heat can be converted into work in a cyclic process. It establishes the concept of entropy, which predicts whether certain processes are forbidden, despite obeying the law of conservation of energy.
The second law of thermodynamics has been expressed in various ways, but it can be understood as stating that the entropy of the universe, as an isolated system, will always increase over time. This implies that the universe will eventually reach a state of maximum disorder, or "heat death", where everything is at the same temperature, and no work can be done. This state is often referred to as the ultimate level of disorder, marking the end of the universe.
The second law also indicates that thermodynamic processes, which involve the transfer or conversion of heat energy, are irreversible. This gives rise to the thermodynamic arrow of time, which states that time always flows in the direction of increasing entropy. This has significant implications for our understanding of time and causality.
While the second law provides valuable insights into the fate of the universe, it is essential to recognize that it operates within the context of isolated systems. The Earth, for example, is not an isolated system, as it constantly receives energy from the sun. Therefore, while the universe as a whole may be moving towards greater disorder, local pockets of increased order, such as the evolution of life, can exist within it.
Additionally, the second law does not provide a precise timeline for the universe's fate. While it predicts that the universe will eventually reach a state of maximum entropy, the timescale for this process is incredibly long, far beyond human comprehension.
In conclusion, the second law of thermodynamics plays a crucial role in understanding the fate of the universe. It implies that the universe will inevitably reach a state of maximum disorder, where all energy is evenly distributed, and no further evolution or work can occur. However, this process will unfold over an extraordinarily long timescale, and local variations, influenced by external factors, can exist within the broader trend towards disorder.
The Dark History of Jim Crow Laws and Their Reach
You may want to see also

The law's role in biology
The second law of thermodynamics states that the entropy of the universe, as an isolated system, will always increase over time. This means that the natural tendency of any isolated system is to degenerate into a more disordered state. This is relevant to biology as living organisms are highly ordered and require a constant input of energy to maintain their state of low entropy.
The second law of thermodynamics can be applied to biological systems by viewing them as open systems, which are not isolated from their surroundings. In this context, the law states that the entropy of a living system can decrease as long as there is an increase of equal or greater magnitude in the entropy of the system's surroundings. This is possible because living systems are not closed systems, and they can exchange matter and energy with their environment.
For example, a living cell builds complex molecules from a mixture of small amino acids or nucleotides. This process seems to contradict the second law of thermodynamics, as it involves increasing order and decreasing entropy within the cell. However, the second law applies only to closed systems, which neither gain nor lose matter or energy. A living cell is an open system that has inputs and outputs of matter and energy. It takes in food molecules as an input of matter and energy, and it produces waste and by-products that are not useful energy sources.
The second law of thermodynamics also has implications for the evolution of life. Some critics claim that evolution violates the second law because it leads to increased organisation and complexity. However, this law only applies to isolated systems, and the Earth is not an isolated or closed system. The Earth receives a constant influx of energy from the Sun, which increases the overall entropy of the universe while allowing for the development of more organised and complex life forms on Earth.
Exploring Legal Differences: English Law in Scotland
You may want to see also

The law's relationship with evolution
The second law of thermodynamics states that the entropy of the universe will always increase over time. This means that the universe will tend towards a state of maximum disorder. However, this law only applies to isolated systems, and the Earth is not an isolated system. The Earth receives a constant input of energy from the Sun, which allows for the formation of complex, ordered structures that decrease the entropy of the Earth. This process of decreasing local entropy is what drives evolution.
The second law of thermodynamics states that the entropy of the universe will always increase over time. This is often referred to as the "arrow of time", indicating that time itself is asymmetric with respect to the order of an isolated system. The law can be stated as:
> The state of entropy of the entire universe, as an isolated system, will always increase over time. The second law also states that the changes in the entropy in the universe can never be negative.
This means that the universe will tend towards a state of maximum disorder. However, this law only applies to isolated systems, and the Earth is not an isolated system. The Earth receives a constant input of energy from the Sun. This external source of energy allows for the formation of complex, ordered structures that decrease the entropy of the Earth.
The formation of these complex, ordered structures is what drives evolution. Evolution can be understood as the emergence of self-replicating dissipative structures that, through natural selection, become increasingly efficient at degrading free energy. Organisms are able to maintain their internal order by consuming free energy and creating more disorder in their environment. This process of decreasing local entropy is what drives evolution.
While the second law of thermodynamics may seem to contradict the process of evolution, it actually provides a necessary condition for evolution to occur. The constant increase in entropy provides a "fitness measure" that drives evolutionary change. Without entropy and the natural tendency for things to decay, there would be no selection, and no evolution.
How Momentum is Conserved in the Universe
You may want to see also

The law's applications
The second law of thermodynamics has a wide range of applications, from biology to cosmology.
Biology
Evolution does not violate the second law of thermodynamics, despite the increase in complexity and organisation that it entails. This is because the second law only applies to isolated systems, and the Earth is not an isolated system. The Earth receives a constant influx of energy from the Sun, and so the universe as a whole becomes more disorganised as the Sun releases energy and becomes disordered.
The second law also plays a role in many biological systems. In food chains, energy escapes as heat between trophic levels, with consumers gaining only a small percentage of the energy stored in their food. On the cellular level, the energy required to maintain the complex structure of a cell increases entropy in the outside environment.
Cosmology
The second law of thermodynamics also has implications for the fate of the universe. It predicts that the universe will eventually reach a state of 'heat death', in which everything is at the same temperature and no work can be done. This is the ultimate level of disorder.
Engines
The second law of thermodynamics dictates the amount of work that can be produced by a transfer of heat. This is often applied to various types of engines. For example, the law describes the amount of work that can be produced by the cycle of increasing and decreasing temperatures in a heat engine.
Everyday Examples
There are also many everyday examples that illustrate the second law of thermodynamics. When a hot object is placed in a room, it quickly spreads heat energy in all directions. When water in a dish is placed on a counter, it eventually evaporates, with the individual molecules spreading out into the surrounding air. When a car tire is punctured, the air disperses in all directions.
Lemon Law and Leased Vehicles: What You Need to Know
You may want to see also
Frequently asked questions
The second law of thermodynamics states that the state of entropy of the entire universe, as an isolated system, will always increase over time. It also states that the changes in the entropy in the universe can never be negative.
When an ice cube is left at room temperature, it begins to melt. Another example is that we get older and never younger. Rooms that are cleaned will eventually become messy again. These are all examples of the "arrow of time", which is the measurement of disorder within a system.
The second law of thermodynamics can be used to explain the age of the Earth. It was also used by scientists to predict that the universe will eventually end in a "heat death", where everything is at the same temperature and no work can be done.
The second law of thermodynamics posits that the transfer of energy involves some energy being released as heat. This inefficient energy transfer plays a role in many biological systems, such as food chains and cellular processes.