Gas laws are a set of physical laws that model the behaviour of gases, and they have wide-ranging applications in our daily lives. One area where gas laws are particularly relevant is in the automotive industry, especially with the growing popularity of electric vehicles (EVs). Gas laws, such as Boyle's Law, Charles' Law, and Avogadro's Law, play a crucial role in understanding the performance and efficiency of both traditional internal combustion engines and the emerging EV technology. These laws govern the relationship between pressure, temperature, volume, and the amount of gas present, which are essential factors in engine design and fuel efficiency. As the transportation sector is a significant contributor to greenhouse gas emissions, a better understanding of gas laws can inform the development of more sustainable and environmentally friendly vehicles.
What You'll Learn

Gas laws and the combustion engine
Gas laws are a set of physical laws that describe the behaviour of gases. They are based on the relationships between pressure, temperature, volume, and the amount of gas present. These laws are applied in the design of cars, specifically in the design of the combustion engine and its various components.
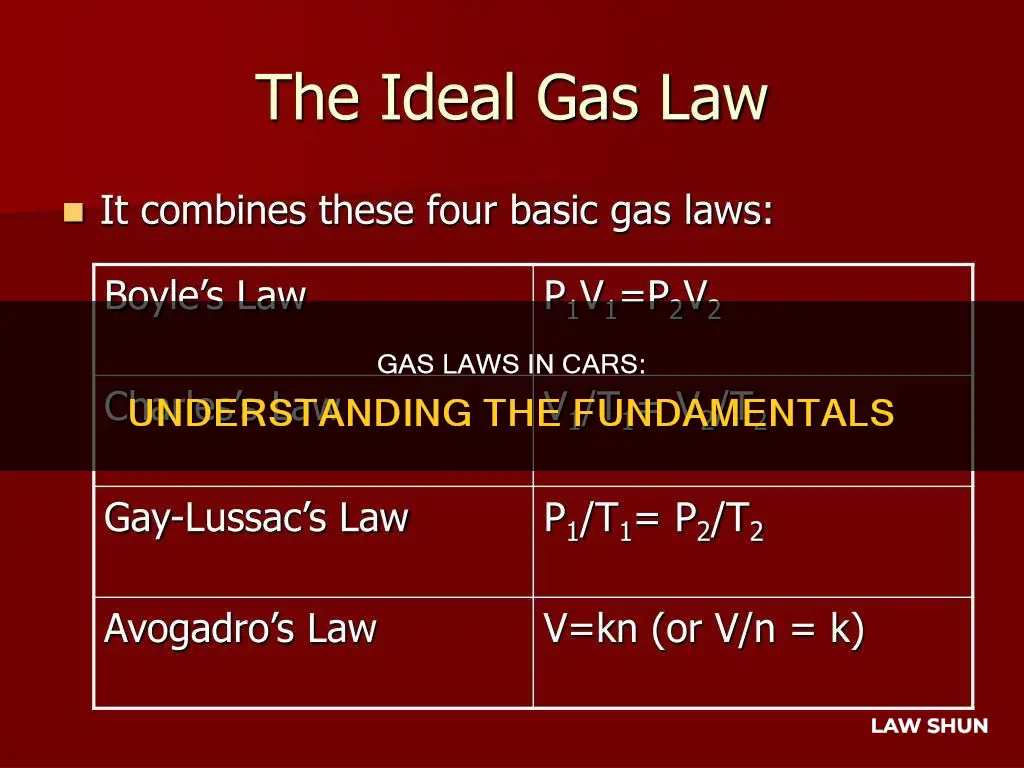
Boyle's Law, which states that the pressure of a gas is inversely proportional to the volume it occupies, is applied in the design of the fuel tank. By understanding the relationship between the pressure and volume of gasoline vapours, engineers can design a tank that is safe and does not leak. This law is also applied to the engine cylinders, where the pressure and volume of the air-fuel mixture change during the combustion process.
Gay-Lussac's Law, which states that the pressure of a gas is directly proportional to its temperature, is applied in the design of the cooling system. By knowing how the temperature and pressure change, engineers can design a system that prevents the engine from overheating. This law is also applied to the engine cylinders, where the temperature and pressure of the air-fuel mixture change during combustion.
Avogadro's Law, which states that the volume of a gas is proportional to the number of moles of the gas, is applied in the design of the fuel injection system. By understanding how the volume and number of moles of gasoline vapours change, engineers can design a system that delivers the correct amount of fuel to the engine. This law is also applied to the engine cylinders, where the number of moles of the air-fuel mixture changes during combustion.
Charles' Law, which states that the volume of a gas is proportional to its temperature, is applied in the design of the engine's intake system. Knowing how the temperature and volume of the air change as it enters the engine helps engineers maximise the efficiency of
Lemon Law and Leased Vehicles: What You Need to Know
You may want to see also

Gas laws and fuel efficiency
The Gas Laws
The gas laws consist of several principles, including:
- Boyle's Law: States that the pressure of a gas is inversely proportional to its volume at a constant temperature. In the context of cars, this law helps explain the behaviour of gases within the engine and fuel system.
- Avogadro's Law: Describes the relationship between the volume of a gas and the number of moles of gas present. This law is essential for understanding the composition of air and fuel mixtures in engines.
- Charles' Law: States that the volume of a gas is directly proportional to its temperature at constant pressure. This law helps explain the expansion and contraction of gases within engines and the impact of temperature changes.
- Gay-Lussac's Law: Explains that for a constant volume, the pressure of a gas is directly proportional to its absolute temperature. This law is crucial for understanding pressure changes in engines and fuel systems.
Fuel Efficiency and Emissions
Fuel efficiency standards, such as the Corporate Average Fuel Economy (CAFE) standards in the United States, aim to reduce oil consumption and greenhouse gas emissions. By applying gas laws, engineers can optimise engine design and fuel injection systems to improve fuel efficiency. This not only saves consumers money at the pump but also reduces global warming pollution.
For example, by understanding the relationship between pressure and volume described by Boyle's Law, engineers can design more efficient fuel injection systems that optimise the air-fuel mixture in the engine's cylinders. Additionally, Charles' Law helps explain the impact of temperature changes on engine performance, allowing engineers to design cooling systems that maintain optimal operating temperatures.
The application of gas laws also extends to emissions regulations. By understanding the behaviour of gases, regulators can set standards for emissions reductions and fuel efficiency improvements. For instance, the Environmental Protection Agency (EPA) in the United States has set standards for greenhouse gas emissions from passenger cars and light-duty vehicles, aiming to reduce harmful air pollutants and improve public health.
In conclusion, gas laws play a critical role in understanding and improving the fuel efficiency of cars. By applying these laws, engineers can design more efficient engines and fuel systems, while regulators can establish standards that reduce emissions and benefit the environment. As fuel efficiency continues to be a critical area of focus for the automotive industry, a strong understanding of gas laws will be essential for further advancements.
Good Samaritan Laws: Do They Protect Doctors?
You may want to see also

Gas laws and emissions standards
The gas laws, including Boyle's Law, Avogadro's Law, and Charles' Law, describe the relationships between pressure, volume, temperature, and the amount of gas present. These laws are essential in understanding the behaviour of gases within car engines, as well as the impact of external factors such as temperature and pressure changes.
In the context of emissions standards, gas laws play a crucial role in reducing air pollutants and improving fuel efficiency. By understanding the principles of gas laws, engineers and scientists can design more efficient engines and emission control systems. For example, Boyle's Law, which states that the pressure of a gas is inversely proportional to its volume, can be applied to design more efficient combustion chambers and optimise fuel injection systems.
Additionally, gas laws are considered when setting emissions standards for vehicles. The Environmental Protection Agency (EPA) in the United States, for instance, has established stringent regulations for greenhouse gas emissions from passenger cars, light-duty trucks, and medium-duty vehicles for model years 2027 through 2032. These standards aim to reduce harmful air pollutants, such as smog and soot, while also improving fuel efficiency and reducing maintenance costs for drivers.
The application of gas laws in emissions standards is not limited to on-road vehicles. The EPA has also issued regulations for non-road vehicles and engines, as well as heavy-duty trucks, to reduce nitrogen oxide (NOx) emissions, which contribute significantly to ground-level air pollution. By leveraging the principles of gas laws, these standards aim to reduce NOx emissions from heavy-duty vehicles by almost 50% by 2045, resulting in significant health benefits.
Furthermore, gas laws are considered in the development of alternative fuel sources and technologies. For example, in the case of electric vehicles, gas laws may influence the design of batteries and energy storage systems, as well as the efficiency of electric motors. Similarly, for hydrogen fuel cell vehicles, gas laws play a crucial role in understanding the behaviour of hydrogen gas and optimising its utilisation.
In conclusion, gas laws are integral to the development and enforcement of emissions standards in the automotive industry. By applying the principles of gas laws, regulators, engineers, and scientists can work towards reducing air pollutants, improving fuel efficiency, and promoting the adoption of cleaner and more sustainable transportation technologies.
Mendel's Law: Segregation in Dihybrid and Monohybrid Crosses
You may want to see also

Gas laws and climate change
Highway vehicles, such as cars, release about 1.5 billion metric tons of GHGs into the atmosphere annually, with carbon dioxide (CO2) being the primary culprit. The combustion of gasoline in car engines leads to the separation of carbon and hydrogen atoms. Carbon combines with oxygen to form CO2, while hydrogen combines with oxygen to form water vapour. This process results in the production of approximately 20 pounds of CO2 for every gallon of gasoline burned.
The accumulation of CO2 and other GHGs, such as methane (CH4), nitrous oxide (N2O), and hydrofluorocarbons (HFCs), in the Earth's atmosphere is causing a rise in global temperatures, leading to climate change. GHG emissions from transportation account for about 28% of total U.S. greenhouse gas emissions, making it the largest contributor to GHG emissions in the country.
To combat this issue, governments and organizations like the Environmental Protection Agency (EPA) have implemented regulations and standards to reduce GHG emissions from vehicles. These regulations include setting GHG emissions and fuel economy standards, promoting the use of renewable and low-carbon fuels, improving fuel efficiency, and encouraging the development of clean car technologies. By adhering to these standards, it is projected that GHG emissions from light-duty vehicles will be significantly reduced over the lifetimes of the vehicles sold between 2012 and 2025.
Additionally, individuals can play a role in mitigating climate change by choosing fuel-efficient vehicles, improving fuel economy, opting for low-carbon fuels or electric vehicles, and reducing their overall vehicle usage. These collective efforts are crucial in addressing the impact of gas laws on climate change and working towards a more sustainable future.
Stand Your Ground: Home Defense and Beyond
You may want to see also

Gas laws and air quality
Boyle's Law, which states that the pressure of a gas is inversely proportional to its volume, helps explain the effects of altitude on gases in enclosed spaces, such as the impact of ascending to higher altitudes on the volume of air in a car tyre or a sealed bag of chips. This law also has clinical applications, such as in hyperbaric therapy and the prevention of barotrauma in divers.
Avogadro's Law, which relates the volume of a gas to the number of moles, is essential for understanding the composition of air and the behaviour of gases at a molecular level. This law helps determine the amount of dissolved gas in a liquid, which has implications for fuel efficiency and emissions.
Charles' Law, stating that the volume of a gas is directly proportional to its temperature, provides insights into the impact of temperature changes on gas volume. This is particularly relevant for understanding the expansion and contraction of air in car engines and the behaviour of gases in vehicle emissions.
The ideal gas law, a combination of Boyle's Law, Charles' Law, Gay-Lussac's Law, and Avogadro's Law, offers a comprehensive framework for analysing gases. It considers the number of moles of gas, the ideal gas constant, absolute temperature, pressure, and volume. This law is applied in various automotive contexts, such as calculating the volume of oxygen available from a cylinder or determining the size and number of cylinders needed for a ventilated patient.
Gas laws also play a crucial role in regulating greenhouse gas emissions from vehicles. Transportation is a significant contributor to greenhouse gas emissions, with the burning of fossil fuels like gasoline and diesel releasing carbon dioxide and other greenhouse gases into the atmosphere. Regulatory bodies, such as the US Environmental Protection Agency (EPA), have implemented standards and regulations to reduce these emissions, aiming to improve fuel efficiency and mitigate climate change.
In summary, gas laws provide a scientific foundation for understanding and managing air quality in the automotive context. They guide the development of more efficient and environmentally friendly vehicles, inform regulatory standards, and help raise awareness about the impact of individual choices on air pollution. By applying these laws, engineers, scientists, and policymakers can work towards creating cleaner and more sustainable transportation systems.
Undocumented Immigrants: Are They Covered by Nondiscrimination Laws?
You may want to see also
Frequently asked questions
Boyle's Law states that the pressure of a fixed mass of gas is inversely proportional to its volume, if its temperature is kept constant. This means that as the volume of a gas increases, its pressure decreases, and vice versa. This principle can be applied to the air inside a car tire. When a car is driven, the air inside the tire is heated and its pressure increases. If the volume remains constant, the pressure inside the tire will increase.
Gas laws describe the relationship between pressure, volume, and temperature of gases. When fuel is burned in a car engine, it undergoes combustion, which increases the temperature and pressure of the gas. This can be explained by Charles' Law, which states that the volume of a gas is proportional to its temperature. As the temperature of the gas increases, so does its volume, assuming the pressure remains constant.
Gas laws, such as Boyle's Law and Charles' Law, can influence the performance of car engines by affecting the pressure and volume of gases within the engine. By understanding these laws, engineers can design engines that optimize the combustion process and improve engine efficiency.
Gas laws can be applied to understand the emission of pollutants from car engines. For example, the increase in pressure and temperature due to combustion can lead to higher emissions of pollutants. Additionally, the gas laws can help regulate emissions by setting standards for fuel efficiency and emission levels, as seen in California's efforts to reduce greenhouse gas emissions from vehicles.







