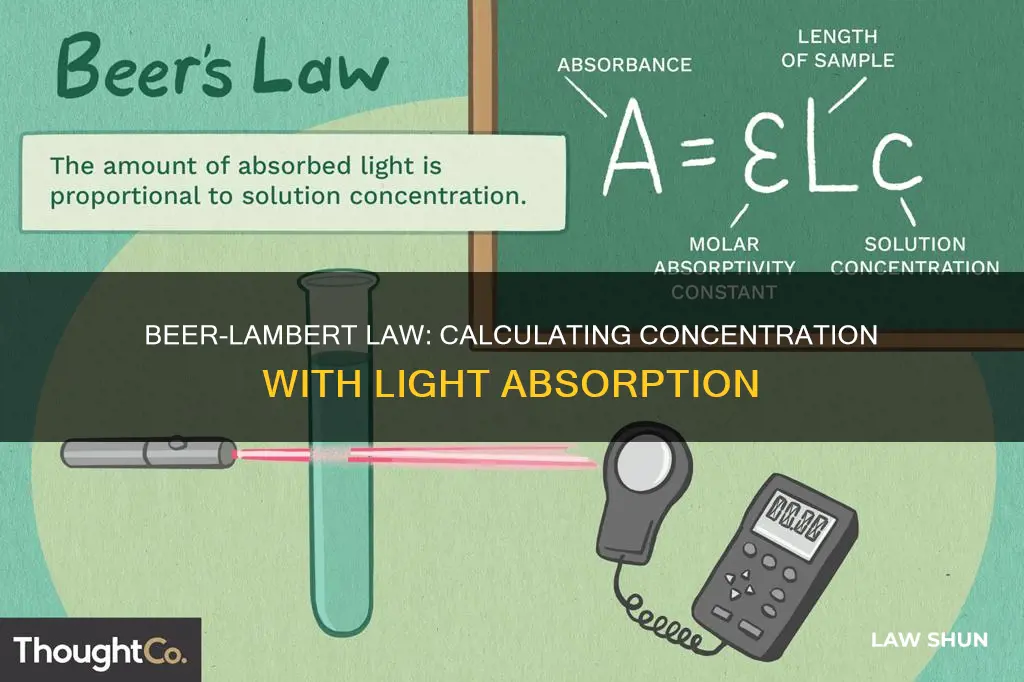
Beer's Law, also known as Beer-Lambert Law, is a fundamental concept in chemistry that relates the concentration of a solution to the attenuation of light as it passes through. In simple terms, it explains how the concentration of a substance affects the amount of light absorbed or transmitted. This law is commonly applied in chemistry to determine the concentration of a solution using absorbance measurements. By utilising tools like a colorimeter or a spectrophotometer, the concentration of a solution can be calculated. The basic equation for Beer's Law is expressed as A = εmCl, where A represents absorbance, εm is the molar extinction coefficient, C is the concentration, and l is the path length of 1 cm.
| Characteristics | Values |
|---|---|
| What is Beer's Law? | A law that gives the relationship between the concentration of a solution and the attenuation of light as it passes through the solution. |
| Beer's Law Equation | A = εmCl |
| Variables | A = absorbance, εm = molar extinction coefficient, C = concentration, l = path length of 1 cm |
| Graphing Method | Plotting absorbance vs. concentration of known solutions to create a standard curve. |
| Proportionality Approach | The ratio of concentrations is proportional to the ratio of absorbances. |
| Molar Absorptivity | The probability of an electronic transition. |
What You'll Learn
- Using a graph to compare the absorbance of an unknown sample to known solutions
- Calculating the concentration of a solution from absorbance
- Using a colorimeter to measure the absorbance of a solution
- Determining the identity of an unknown substance by its molar absorptivity
- The importance of the shape of the container

Using a graph to compare the absorbance of an unknown sample to known solutions
To calculate the concentration of a solution using Beer's Law, you need to use a graph plotting Absorbance vs. Concentration of known solutions. This is known as a standard curve. Once you have this, you can compare the absorbance value of an unknown sample to find its concentration.
The standard curve is generated by measuring the absorbance of a series of known solutions. The independent variable, concentration, is plotted on the x-axis, and the dependent variable, absorbance, is plotted on the y-axis. By adding a line of best fit to the data points, you can determine the equation of the line, which is usually in the form of y=mx + b.
The equation for Beer's law is: A = εmCl, where A is the absorbance, εm is the molar extinction coefficient, C is the concentration, and l is the path length of 1 cm.
To find the concentration of an unknown solution, you can use the equation of the line derived from the standard curve. The concentration (C) can be calculated using the formula: C = (A-b)/εm, where A is the absorbance of the unknown sample, b is the y-intercept, and εm is the slope of the line.
For example, let's say you have an unknown sample with an absorbance (A) of 6.00. From the standard curve, you determine that the slope (m) is 8 and the y-intercept (b) is 0. By substituting these values into the equation, you get 6.00 = 8*C + 0, which simplifies to 6/8 = C. Solving for C, you find that the concentration of the unknown solution is 0.75, or 75% of the original stock solution.
Truancy Laws: Do They Apply to 17-Year-Olds?
You may want to see also

Calculating the concentration of a solution from absorbance
To calculate the concentration of a solution from absorbance, you can apply Beer's Law, also known as the Beer-Lambert Law. This law relates the concentration of a solution to the attenuation of light as it passes through the solution.
The equation for Beer's Law is: A = εmCl
Where:
- A = absorbance
- Εm = molar extinction coefficient
- C = concentration
- L = path length of light through the solution (usually in cm)
To use this equation, you need to determine the absorbance of light as it passes through the solution. This can be done using a spectrophotometer, which measures the intensity of light before and after it passes through the solution.
Next, you need to know the path length of the light through the solution, which is usually provided or can be measured.
To calculate the concentration, you can rearrange the equation as: C = (A-b)/εm
Here, 'b' is the y-intercept of the standard curve, which is obtained by plotting known concentrations (x-axis) against their corresponding absorbance values (y-axis). By subtracting the y-intercept from the absorbance and dividing by the slope of the curve, you can determine the concentration of your solution.
This method is particularly useful for analytical chemistry applications, such as determining the concentration of unknown samples or identifying substances by their molar absorptivity.
Whistleblower Law: Contractor Rights in Pennsylvania
You may want to see also

Using a colorimeter to measure the absorbance of a solution
A colorimeter is a device used in colorimetry that measures the absorbance of specific wavelengths of light by a solution. It is commonly used to determine the concentration of a known solute in a given solution by applying Beer's Law. The essential parts of a colorimeter are:
- A light source (often a low-voltage filament lamp)
- An adjustable aperture
- A set of coloured filters
- A cuvette to hold the working solution
- A detector (usually a photoresistor) to measure the transmitted light
- A meter to display the output from the detector
The colorimeter was invented in 1870 by Louis J Duboscq. It is based on two fundamental laws of photometry:
- The amount of light absorbed is proportional to the solute concentration present in the solution.
- The amount of light absorbed is proportional to the length and thickness of the solution taken for analysis.
Step 1: Calibration
Before starting the experiment, the colorimeter must be calibrated using standard solutions of the known solute concentration that needs to be determined. Fill the standard solutions into the cuvettes and place them in the cuvette holder of the colorimeter.
Step 2: Directing Light
A light ray of a certain wavelength, specific to the assay, is directed towards the solution. The light passes through a series of different lenses and filters. The coloured light navigates with the help of lenses, and the filter helps to split a beam of light into different wavelengths, allowing only the required wavelength to pass through and reach the cuvette of the standard test solution.
Step 3: Transmittance, Reflection, and Absorption
When the beam of light reaches the cuvette, it is transmitted, reflected, and absorbed by the solution.
Step 4: Photodetector System
The transmitted ray falls on the photodetector system, which measures the intensity of the transmitted light. It converts this measurement into electrical signals and sends them to the galvanometer.
Step 5: Digital Display
The electrical signals measured by the galvanometer are displayed in digital form.
Step 6: Calculating Concentration
The concentration of the substance in the test solution can be calculated using Beer's Law:
- For standard and test solutions, ∈ and l are constant.
- CT is the test solution concentration.
- AT is the absorbance/optical density of the test solution.
- CS is the standard concentration.
- AS is the absorbance/optical density of the standard solution.
Applications of Colorimeters
Colorimeters are used in various fields, including:
- Laboratories and hospitals to estimate biochemical samples such as urine, cerebrospinal fluid, plasma, and serum.
- The manufacturing of paints.
- The textile and food industries.
- Quantitative analysis of proteins, glucose, and other biochemical compounds.
- Testing water quality.
- Determining the concentration of haemoglobin in the blood.
- Tracking the growth of bacterial or yeast colonies.
- Evaluating the colour in bird plumage.
- Quantifying and tracking colour in various foods and drinks, including vegetable products and sugar.
Understanding California Overtime Laws: Part-Time Employee Rights
You may want to see also

Determining the identity of an unknown substance by its molar absorptivity
Beer's Law, also known as the Beer-Lambert Law, relates the concentration of a solution to the attenuation of light as it passes through it. It is used to determine the concentration of a solution by measuring its absorbance.
The Beer-Lambert Law equation is expressed as:
A = εlc
Where:
- A is the absorbance
- Ε (epsilon) is the molar absorptivity or molar absorption coefficient
- L is the path length of the beam of light in the sample
- C is the concentration of the solution
To determine the identity of an unknown substance by its molar absorptivity, you can use the Beer-Lambert Law in conjunction with spectrophotometry techniques. Here's a step-by-step guide:
- Understand the Theory: Start by understanding the underlying theory, including the Beer-Lambert Law equation and the concept of molar absorptivity. Molar absorptivity, also known as the molar extinction coefficient, measures how effectively a chemical species absorbs a specific wavelength of light. It allows for comparisons between different compounds, regardless of variations in concentration or solution length during measurements.
- Prepare a Solution of Known Concentration: Prepare a solution with a known concentration, 'c', in units of molarity or moles per liter. This solution will serve as a reference for your measurements.
- Measure the Path Length: Determine the path length, 'l', which represents the distance the light travels through the solution. Typically, this is measured as the length of the cuvette, the piece that holds the liquid samples in the spectrophotometer. The path length is usually expressed in centimeters.
- Measure Absorbance with a Spectrophotometer: Employ a spectrophotometer to measure the absorbance, 'A', of the solution at a specific wavelength. The spectrophotometer will pass light of a particular wavelength through the solution and detect the amount of light that emerges. The unit for wavelength is typically meters or nanometers (nm). It's important to note that absorbance is unitless.
- Rearrange the Beer-Lambert Law Equation: Rearrange the Beer-Lambert Law equation to solve for molar absorptivity. Using algebra, divide absorbance by the product of length and concentration: ε = A/lc.
- Plug in the Values and Calculate: Substitute the values you obtained for absorbance ('A'), concentration ('c'), and path length ('l') into the rearranged equation. Then, perform the calculations to determine the molar absorptivity.
- Compare with Known Values: Finally, compare the calculated molar absorptivity of your unknown substance with known values for different compounds. This comparison will help you identify the unknown substance based on its unique molar absorptivity.
By following these steps and utilizing the Beer-Lambert Law, you can determine the identity of an unknown substance by analyzing its molar absorptivity in relation to the absorption of light.
Inertia's Law: Universal or Selective Applicability?
You may want to see also

The importance of the shape of the container
The Beer-Lambert law states that the attenuation of light is directly proportional to the concentration of the solution and the length of the light path. The importance of the shape of the container comes into play when considering the length of the light path.
The length of the light path refers to the distance that light travels through the solution. If a very dilute solution of a dye is placed in a cube-shaped container, the light path will be shorter than if the same solution is placed in a long tube. As a result, the absorbance of light will be lower in the cube-shaped container compared to the tube. This is because the light interacts with fewer molecules in the shorter path, resulting in less absorption.
Therefore, when applying Beer's law to calculate concentration, it is crucial to consider the shape and dimensions of the container. The length of the light path directly affects the absorbance value, which is used to determine the concentration of the solution. By ensuring that the container has a consistent shape and dimensions, accurate and reproducible results can be obtained when using Beer's law for concentration calculations.
Additionally, the Beer-Lambert law can be rearranged to calculate the molar absorptivity or molar extinction coefficient, which is a measure of the probability of electronic transition. This value is independent of the concentration and length of the solution, allowing for comparisons between different compounds.
In summary, the shape of the container is important when applying Beer's law because it directly affects the length of the light path, which in turn influences the absorbance and, ultimately, the calculated concentration of the solution.
Lemon Law: Does It Expire After 18 Months?
You may want to see also
Frequently asked questions
Beer's Law, also known as the Beer-Lambert Law, states that the attenuation of light is directly proportional to the concentration of a solution it passes through. The law is expressed as: A = εlc, where A = absorbance, ε = molar absorptivity, l = path length, and c = concentration.
To calculate concentration, you need to measure the absorbance of a solution of known concentration and path length. Then, apply the formula: Concentration = Absorbance / Slope (where the slope is the product of ε and l).
The unit of concentration in Beer's Law is typically expressed in mol/L or M (molarity).
Sure! A = absorbance, which is the ratio of incident light intensity (Io) to transmitted light intensity (I). ε (epsilon) is the molar absorptivity or molar extinction coefficient, representing the probability of electronic transition. l = path length, and c = concentration.
Beer's Law is widely used in analytical chemistry to determine the concentration of samples by measuring absorbance. It also helps identify unknown substances by determining their molar absorptivity.