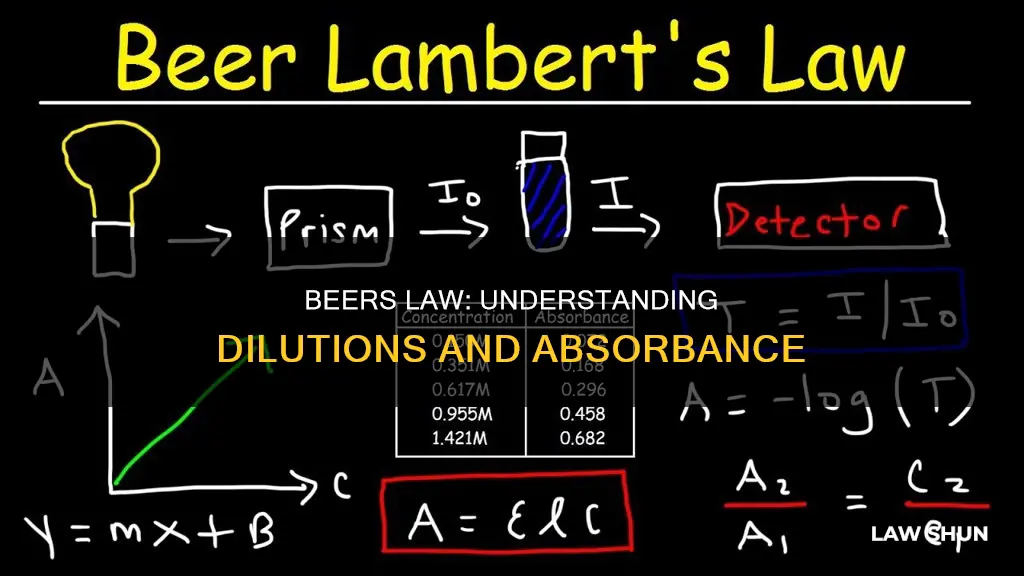
Beer's Law, also known as the Beer-Lambert Law, is an equation that relates light attenuation to the properties of the material through which the light is travelling. The law states that the concentration of a chemical is directly proportional to the absorbance of a solution. In other words, a high-concentration solution absorbs more light, and a low-concentration solution absorbs less light. This relationship is expressed in the following equation: A = εLc, where A is the amount of light absorbed by a sample at a specific wavelength, ε is the extinction coefficient at the molar level, L is the length of time that light travels through the solution, and c is the concentration of the absorbing species. This law is particularly useful in the fields of chemistry, physics, and meteorology. For example, Beer's Law can be used to determine the concentration of chemical solutions, assess oxidation, and monitor polymer deterioration.
What You'll Learn

How does Beer's Law relate to light attenuation?
Beer's Law, also known as the Beer-Lambert Law, is an equation that relates light attenuation to the properties of the material through which light is travelling. The law states that the concentration of a chemical is directly proportional to the absorbance of a solution. In other words, a high-concentration solution absorbs more light, whereas a low-concentration solution absorbs less light.
The Beer-Lambert Law is expressed as:
> A = εlc
Where:
- A is the amount of light absorbed by the sample at a specific wavelength
- Ε is the extinction coefficient at the molar level
- L is the length of time that light travels through the solution
- C is the concentration of the absorbing species
The Beer-Lambert Law can be used to determine the concentration of a chemical species in a solution. By measuring the amount of light absorbed by a sample at a specific wavelength, the concentration of the chemical can be calculated. This is done using a colorimeter or spectrophotometer.
The law assumes a straight-line relationship between absorbance and concentration for dilute solutions. It is important to note that Beer's Law only holds true at absorbance values between 0.1 and 1. Outside of this range, the relationship between absorbance and concentration is no longer linear.
The Beer-Lambert Law is used in various fields, including chemistry, physics, and meteorology. In chemistry, for example, it is used to determine the concentration of chemical solutions, assess oxidation, and monitor polymer deterioration.
Usury Laws: Florida's Business Loan Interest Rate Limits
You may want to see also

How does Beer's Law apply to spectrophotometers?
Beer's Law, also known as the Beer-Lambert Law, is an equation that relates light attenuation to the properties of the material through which the light is travelling. The law states that the concentration of a chemical is directly proportional to the absorbance of a solution.
A spectrophotometer is an instrument used to measure the amount of light absorbed by a sample. It does this by directing light into the sample and measuring the amount of light that is transmitted. The power of the light source will be larger than the power of the transmitted light because the molecules in the sample will absorb some of the light.
The Beer-Lambert Law can be used to determine the concentration of a chemical species in a solution. The law is expressed as:
> A = εlc
Where:
- A is the amount of light absorbed by the sample at a specific wavelength
- Ε is the extinction coefficient at the molar level
- L is the length of the light path
- C is the concentration of the absorbing species
The Beer-Lambert Law assumes that the absorbance of a solution is directly proportional to its concentration. This means that as the concentration of a solution increases, the absorbance of the solution also increases.
The law can be used to determine the concentration of an unknown substance by measuring the absorbance of the solution and then using the Beer-Lambert Law to calculate the concentration. This is particularly useful in fields such as chemistry, where it can be used to determine the concentration of chemical solutions, assess oxidation, and monitor polymer deterioration.
For example, let's say we have a solution with a maximum absorbance of 275 nm, a molar absorptivity of 8400 M^-1 cm^-1, and a cuvette diameter of 1 cm. We use a spectrophotometer to measure the absorbance of the solution and find that A = 0.70. To calculate the concentration of the solution, we can use the Beer-Lambert Law:
> 0.70 = (8400 M^-1 cm^-1) * (1 cm) * c
Dividing both sides of the equation by [(8400 M^-1 cm^-1) * (1 cm)], we get:
> c = 8.33 x 10^-5 mol/L
So, the concentration of the solution is 8.33 x 10^-5 mol/L.
Ohm's Law and Light Bulbs: What's the Connection?
You may want to see also

How to calculate the concentration of a chemical species in a solution
Beer's Law states that the absorbance of a solution is directly proportional to the concentration of the solute in that solution. The Beer-Lambert Law, the Lambert-Beer Law, and the Beer-Lambert–Bouguer Law are all variations of Beer's Law.
The general formula for Beer's Law is:
> A = εLc
Where:
- A is the amount of light the sample absorbs at a specific wavelength
- Ε is the extinction coefficient at the molar level
- L is the length of time that light travels through the solution
- C is the concentration of the absorbing species
To calculate the concentration of a chemical species in a solution, you can rearrange the formula to:
> c = A / (εL)
For example, if you have a solution with a maximum absorbance of 275 nm, a molar absorptivity of 8400 M^-1 cm^-1, and an absorbance of 0.70, you can calculate its concentration as follows:
- Plug the values into the formula: 0.70 = (8400 M^-1 cm^-1) * 1 cm * c
- Divide both sides by (8400 M^-1 cm^-1) * 1 cm to isolate c: c = 8.33 x 10^-5 mol/L
It's important to note that Beer's Law assumes ideal conditions and is most accurate for dilute solutions. Additionally, the relationship between absorbance and concentration is linear only between absorbance values of 0.1 and 1.
US Laws and Non-Citizens: Who's Affected and How?
You may want to see also

How to use Beer's Law to identify the type of poison
Beer's Law is an important concept in chemistry, physics, and meteorology, and it can be used to identify the type of poison in a given sample. Here's a step-by-step guide on how to use Beer's Law for poison identification:
Understanding Beer's Law
Beer's Law, also known as the Beer-Lambert Law or the Lambert-Beer Law, states that the concentration of a chemical is directly proportional to the absorbance of a solution. In simple terms, it means that the more concentrated a solution is, the more light it will absorb. This relationship is expressed by the equation:
> A = εLc
Where:
- A is the amount of light absorbed by the sample at a specific wavelength.
- Ε (epsilon) is the extinction coefficient at the molar level.
- L is the length of the path the light travels through the solution.
- C is the concentration of the absorbing species.
Preparing Standard Solutions
To identify an unknown poison, you will need to create a series of standard solutions with known concentrations of potential poisons. Dilute a stock solution of each suspected poison and measure the percent transmittance of each solution at its characteristic wavelength. This wavelength is chosen by examining the absorption spectrum of the solution. The solution's colour will help determine the complementary colour of light it is absorbing.
Creating a Calibration Curve
Plot the absorbance of the blank and standard solutions against their corresponding concentrations. This plot is known as a calibration or standard curve. The points should form a straight line. Use the equation of the best-fit line to determine the unknown concentration. The equation will be in the form:
> Absorbance = m * Concentration + c
Where m is the slope of the line and c is the y-intercept.
Measuring Absorbance of the Unknown Poison
Measure the absorbance of the unknown poison solution at the same wavelength as the standard solutions. This can be done using a spectrophotometer or a colorimeter.
Identifying the Poison
Substitute the measured absorbance value into the calibration curve equation to solve for the concentration of the unknown poison. Compare this concentration with the known concentrations of the standard solutions to identify the type of poison.
Example Calculation
Let's say you have an unknown solution with an absorbance of 0.70 at a wavelength of 275 nm. You suspect it might be guanosine, which has a molar absorptivity (ε) of 8400 M-1cm-1. The path length (L) is 1 cm. Using Beer's Law:
70 = (8400 M-1cm-1) * 1 cm * c
Now, divide both sides by (8400 M-1cm-1) * 1 cm to isolate c:
C = 8.33 x 10^-5 mol/L
So, the concentration of the unknown solution is 8.33 x 10^-5 mol/L. You can now compare this concentration with your standard solutions to identify the poison.
Ohms Law and Switches: What's the Verdict?
You may want to see also

How to use Beer's Law to calculate the concentration of a diluted solution
Beer's Law states that the absorbance of a solution is directly proportional to the concentration of the solute in that solution. The law is represented by the equation:
A = εbc
Where:
- A = absorbance of the solution
- Ε = molar absorptivity or extinction coefficient of the solute
- B = path length of light through the solution
- C = concentration of the solution
To use Beer's Law to calculate the concentration of a diluted solution, follow these steps:
- Determine the absorbance (A) of the diluted solution using a spectrophotometer. This will involve measuring the intensity of light passing through a reference cell (Io) and the intensity of light passing through the sample cell (I). The absorbance is then calculated as A = log10(Io⁄I).
- Measure the path length (b): This is the width of the cuvette or container holding the solution.
- Find the molar absorptivity (ε): This is a constant specific to each solute and wavelength. It can be determined experimentally by measuring the absorbance of a solution with a known concentration.
- Rearrange the equation to solve for concentration (c): Rearrange the equation to the following form: c = A ⁄ (εb).
- Plug in the values you have determined for A, ε, and b into the equation and calculate the concentration.
It is important to note that Beer's Law is most accurate for solutions with absorbance values between 0.1 and 1. Outside this range, the relationship between absorbance and concentration may not be linear. Additionally, the units of your values should be consistent, typically in M (moles/litre) for concentration and cm for path length.
Understanding Moratorium Law: Who Does It Affect?
You may want to see also
Frequently asked questions
Beer's Law is an equation that relates light attenuation to the properties of the material through which the light is travelling. It is used to determine the concentration of a chemical species in a solution.
Beer's Law states that the absorbance of a solution is directly proportional to its concentration. Therefore, if you dilute a solution, its absorbance will decrease, and you can use this decrease in absorbance to calculate the new concentration.
The equation for Beer's Law is A = εlc, where A is the absorbance, ε is the molar absorptivity or extinction coefficient, l is the length of the light path, and c is the concentration of the solution.
To calculate the concentration of a diluted solution, first measure the absorbance of the diluted solution. Then, use the equation A = εlc, rearranged to solve for c: c = A / (εl).
Beer's Law is most accurate for solutions with absorbance values between 0.1 and 1. Outside this range, the relationship between absorbance and concentration may not be linear, so the calculations may be less accurate.







