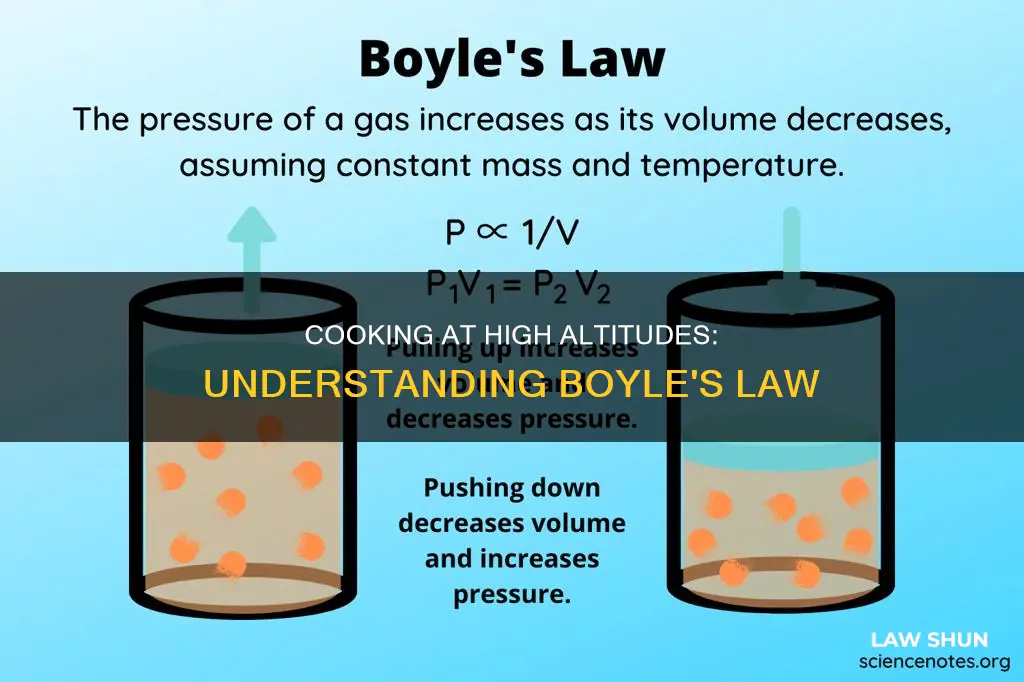
Cooking at high altitudes requires some special considerations due to the thin air and lower air pressure. One of the major physical forces involved in baking at high altitudes is Boyle's Law, which describes the relationship between the pressure and volume of a confined gas. According to Boyle's Law, as volume increases, the pressure of a gas decreases proportionally, and vice versa, when the temperature is held constant. This law can be applied to cooking methods such as boiling, simmering, and baking, where changes in temperature and pressure can affect the outcome of the cooked product.
| Characteristics | Values |
|---|---|
| Air pressure at high altitudes | Lower |
| Effect of air pressure on cooking temperature | Inversely proportional |
| Effect of high altitude on cooking temperature | Decrease |
| Effect of high altitude on cooking time | Increase |
| Effect of high altitude on boiling temperature of water | Decrease |
| Effect of high altitude on moisture in food | Loss of moisture |
| Effect of high altitude on leavening gases in bread and cakes | More expansion |
What You'll Learn

Cooking at high altitudes requires adjustments to time and temperature
At high altitudes, the air pressure is lower, which means that the cooking temperature will also be lower. To compensate for this, either the cooking temperature must be increased or the cooking time must be extended.
The decrease in air pressure at high altitudes affects the preparation of food in several ways. Firstly, water and other liquids evaporate faster and boil at lower temperatures. This means that foods that are prepared by boiling or simmering will cook at a lower temperature, and it will take longer for them to cook. To compensate for the lower boiling point of water, the cooking time must be increased. Turning up the heat will not help to cook food faster, as water cannot exceed its boiling point, unless a pressure cooker is used.
Secondly, high-altitude areas tend to have low humidity, which can cause the moisture in foods to evaporate more quickly during cooking. To retain moisture, foods should be covered during cooking.
Additionally, at high altitudes, the leavening gases in breads and cakes expand more. Therefore, it is important to reduce the amount of leavening agents such as baking powder or baking soda when baking at high altitudes. Reducing the amount of air introduced into batters is another crucial adjustment that must be made. This can be achieved by under-beating eggs.
In summary, when cooking at high altitudes, adjustments to both time and temperature are necessary to compensate for the decrease in air pressure. These adjustments ensure that food is cooked thoroughly and safely, while also maintaining its desired texture and moisture content.
Sunshine Laws: Do They Shine on Property Owners Associations?
You may want to see also

Liquids evaporate faster and boil at lower temperatures
At high altitudes, the atmosphere is less dense, resulting in lower atmospheric pressure. This decrease in pressure affects cooking by causing liquids to evaporate more quickly and boil at lower temperatures. The reduced pressure also impacts the leavening gases in baked goods, causing them to expand more than they would at sea level.
The decrease in boiling temperature with increasing altitude can be observed by looking at the boiling point of water. For every 500-foot increase in elevation, the boiling point of water decreases by almost 1 °F. For example, at 7,500 feet above sea level, water boils at approximately 198 °F instead of 212 °F. This decrease in boiling temperature means that foods prepared by boiling or simmering will cook at a lower temperature and may take longer to reach the desired level of doneness.
To compensate for the lower boiling point, the cooking time must be increased. Simply increasing the heat will not solve the problem, as water cannot exceed its boiling point, regardless of the temperature. Using a pressure cooker can help address this issue by increasing the atmospheric pressure and, consequently, the boiling temperature of water.
In summary, at high altitudes, liquids evaporate faster and boil at lower temperatures due to reduced atmospheric pressure. This effect is described by Boyle's law and has important implications for cooking, particularly in terms of cooking time and temperature adjustments.
Michelle's Law: Still Relevant or Outdated?
You may want to see also

Leavening gases in dough expand more
To compensate for the increased expansion of leavening gases at high altitudes, it is recommended to decrease the amount of leavening agents used. This can help prevent baked goods from rising too rapidly and then collapsing. The recommended reduction is between 15 to 25% of the amount of leavening agent that would typically be used at sea level.
In addition to reducing the amount of leavening agent, other adjustments can be made to recipes when baking at high altitudes. These adjustments aim to address the challenges posed by the lower air pressure and drier air, which can affect the texture, moisture content, and shape of baked goods.
One adjustment is to increase the oven temperature by 15-25 degrees Fahrenheit, or 25% of the standard temperature. This helps to set the structure of the baked goods before they have a chance to dry out or expand too much. However, it is important to also decrease the baking time by 5 to 8 minutes per 30 minutes of baking time to prevent overcooking.
Another adjustment is to increase the amount of liquid in the recipe. The drier air at higher altitudes can cause baked goods to become dry and crumbly. Adding extra liquid helps to prevent this issue. It is generally recommended to add an extra 1-2 tablespoons of liquid at 3,000 feet and an additional 1 to 1.5 teaspoons for every additional 1,000 feet of elevation.
Additionally, it is suggested to decrease the amount of sugar in the recipe by about 1 to 3 tablespoons per cup. Sugar tends to crystallize more quickly at higher altitudes, resulting in a drier end product. Reducing the amount of sugar helps to retain moisture and prevent the baked goods from becoming overly sweet.
Furthermore, it is advisable to decrease the amount of fat in the recipe by about 1 to 2 tablespoons. The lower air pressure at high altitudes causes fats to break down gluten faster, which can lead to a greasy or tender texture. Reducing the fat content helps to maintain the structural strength of the baked goods.
Finally, using high-protein flour, such as bread flour or all-purpose flour with added gluten, can provide more structure and stability to baked goods at high altitudes. The higher protein content helps to improve the rise and texture of the final product. Substituting about 2 tablespoons of high-protein flour for every cup of all-purpose flour is generally recommended.
Family Law Statutes: Civil Cases in California
You may want to see also

Gases are kept in solution by higher pressure
This law is particularly relevant when cooking at high altitudes, where the atmospheric pressure is lower. When cooking at high altitudes, water and other liquids evaporate faster and boil at lower temperatures. This is because, as atmospheric pressure decreases, the boiling point of water also decreases. For example, at 7,500 feet, water boils at around 198 °F, compared to 212 °F at sea level.
The lower atmospheric pressure at high altitudes also affects the way gases behave in baked goods. Leavening gases in breads and cakes expand more, causing the dough to rise. This is because gases are kept in solution by higher pressure, so at high altitudes, gases dissolved in the dough escape more rapidly and can ruin the texture of the baked goods.
To compensate for the lower pressure at high altitudes, cooks must make adjustments to their recipes. When baking at high altitudes, it is important to reduce the amount of leavening agents such as baking powder or soda, and to under-beat eggs to reduce the amount of air introduced into the batter. These adjustments help to prevent the gases from escaping too quickly and ensure that the baked goods rise properly.
In addition to these changes, cooks at high altitudes may also need to increase the moisture and sugar content in their recipes, as flours tend to dry out more quickly in low-pressure environments. By making these adjustments, cooks can ensure that their baked goods turn out properly, even in the lower-pressure conditions of high altitudes.
HIPAA Laws: Do They Apply to Businesses?
You may want to see also

Oven temperatures are unaffected by altitude changes
At altitudes above 3,000 feet, the preparation of food may require changes in time, temperature, or recipe. This is due to the lower atmospheric pressure resulting from a thinner blanket of air. At sea level, the air pressure is 14.7 pounds per square inch, while at 5,000 feet it is 12.3 pounds, and at 10,000 feet, it drops to 10.2 pounds. This decrease in pressure impacts food preparation in two significant ways. Firstly, water and other liquids evaporate faster and reach boiling points at lower temperatures. Secondly, leavening gases in breads and cakes expand more.
The boiling point of water decreases with increasing altitude. For every 500-foot increase in elevation, the boiling point of water drops by almost 1 °F. Therefore, at 7,500 feet, water boils at approximately 198 °F. As a result, foods prepared by boiling or simmering will cook at a lower temperature, and it will take longer to cook.
High-altitude areas often have low humidity, causing moisture in foods to evaporate more quickly during cooking. To retain moisture, it is recommended to cover foods during and after cooking. While oven temperatures remain constant regardless of altitude, cooking methods such as boiling, simmering, or braising may require up to 25% more cooking time at higher altitudes.
When cooking meat and poultry at high altitudes, adjustments in both time and moisture are typically necessary. This is particularly true for meat cooked by simmering or braising. Depending on the density and size of the pieces, meats and poultry cooked by moist heat may take up to one-fourth more cooking time at 5,000 feet.
In summary, while high altitudes present unique challenges for cooking, oven temperatures remain unaffected by these changes. It is important to consider the impact of altitude on cooking times and temperatures for specific cooking methods and make adjustments as necessary.
Inverse Square Law: Understanding Magnetic Force Laws
You may want to see also
Frequently asked questions
Boyle's Law states that the pressure exerted by a given mass of an ideal gas is inversely proportional to the volume it occupies if the temperature and amount of gas remain unchanged within a closed system. In other words, as altitude increases and atmospheric pressure decreases, the boiling point of water decreases. This means that food will take longer to cook.
Gases are light and they rise. The temperature and pressure determine whether gases will remain dissolved or dissipate. At high altitudes, the pressure is lower than at sea level, and gases dissolved in dough escape more rapidly, which can ruin the texture of baked goods.
When cooking at high altitudes, it is important to reduce the amount of leavening, such as baking powder or soda, and slightly increase moisture as flours tend to dry out. It is also crucial to increase the cooking time to compensate for the lower boiling point of water.