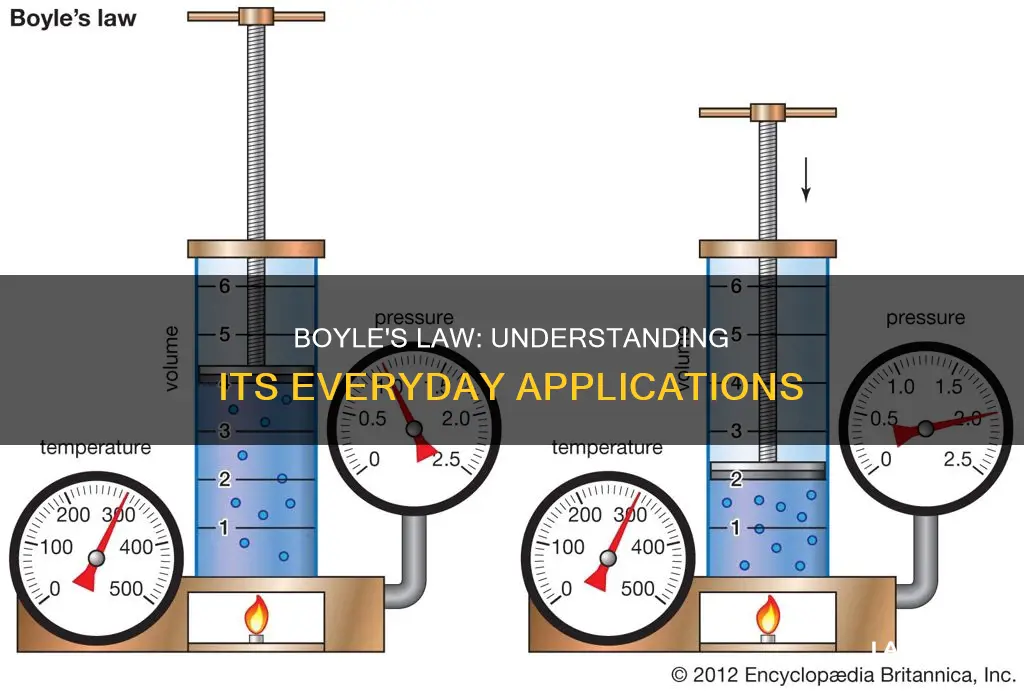
Boyle's Law, discovered by Robert Boyle in 1662, states that for a fixed amount of gas, volume is inversely related to pressure when held at a constant temperature. This means that as volume increases, pressure decreases, and vice versa. This law has several applications in everyday life, from filling up a tire with air to using a syringe or opening a carbonated drink. It also explains the phenomenon of the bends in scuba divers and the importance of a slow ascent from the ocean depths to avoid injury or death.
| Characteristics | Values |
|---|---|
| Spray paint | When the spray nozzle is pressed, the gas starts to get out of the container, the boiling state starts, the liquefied gas expands and turns into gas, and the gas presses the paint inside the container. |
| Soda bottle | When the bottle of soda is opened quickly, the gas rushes from everywhere in the form of foam, causing a mess. |
| Diving into deep water | When the diver moves down to the bottom of the water, the pressure increases. Increasing pressure leads to a decrease in volume, and the diver’s blood begins to absorb the nitrogen gas. |
| Syringes | When the plunger is pulled back, the volume within the chamber increases and the pressure decreases, creating a vacuum. When a syringe is empty, the vacuum within the chamber sucks fluid in through the needle. |
| Basketball bouncing | When a basketball hits the ground, the volume decreases and the pressure increases, and the increasing pressure springs the ball back up. |
| Breathing | When a person breathes in, their lung volume increases and the pressure within decreases, and air is drawn into the lungs. |
What You'll Learn

Spray paint cans
The spray cans contain two substances: the paint itself, and a pressurised gas in liquid form. This gas has a boiling point below room temperature, but because the can is sealed, it remains in liquid form.
When the nozzle of the can is pressed, the seal is broken, and the gas begins to boil and expand. This expansion increases the pressure inside the can, forcing the paint out of the nozzle.
This is a simple but powerful application of Boyle's Law, which states that the pressure and volume of a gas are inversely proportional to one another. In other words, as the pressure on a gas increases, its volume decreases, and vice versa.
The spray can utilises this principle to force the paint out of the nozzle. By decreasing the pressure on the gas, its volume increases, and the expanding gas pushes the paint out of the can. This is a great example of how Boyle's Law can be applied in everyday life to perform useful tasks.
English Law in the US: Who Rules?
You may want to see also

Soda bottles
When a soda bottle is filled, it is also pressurized. Much like an aerosol can, when you slowly open the cap, the gas is able to increase its volume and the pressure decreases. Normally, you can let the gas out of a can or bottle without any issues, but if the bottle is shaken and the gas mixes with the liquid, you may have a mess on your hands. This is because the gas, in its rush to escape, brings the foamy liquid with it. The pressure in the bottle goes down, the volume of the gas goes up, and you have a mess to clean up.
Boyle's Law states that for a fixed amount of gas, volume is inversely related to pressure. This means that as volume rises, pressure drops, and vice versa. This is applicable in the respiratory system. When you inhale, your lung volume increases and the pressure within decreases, so air is drawn into your lungs. The opposite happens when you exhale.
Boyle's Law is also important when using a syringe. When fully depressed, the syringe is at a neutral state with no air in the cylinder. When the plunger is pulled back, you are increasing the volume in the container and thus reducing the pressure. The liquid is then drawn up into the syringe because it balances the pressure, making it equal to the pressure outside of the syringe.
Moore's Law: Hard Drive Edition?
You may want to see also

SCUBA diving
As a SCUBA diver descends, the water pressure around them increases, causing the air spaces in their body (ears, sinuses, lungs) and equipment to compress. If a diver were to hold their breath during this descent, the air in their lungs would be at a lower pressure than the surrounding water, and their lungs would be damaged as they attempt to equalise the pressure.
Similarly, as a diver ascends, the pressure decreases and the air in their body and equipment expands. If a diver ascends while holding their breath, the air in their lungs will expand and could cause their lungs to rupture, leading to a pulmonary barotrauma or arterial gas embolism, both of which are life-threatening conditions.
Boyle's Law also explains why divers must ascend slowly. During a dive, a diver's body absorbs nitrogen from the air in the tank. If a diver ascends too quickly, the nitrogen does not have time to off-gas safely and can form bubbles in the blood and tissue, leading to decompression sickness.
Boyle's Law is an essential concept for SCUBA divers to understand, as it helps them anticipate how air will behave during a dive and follow safety guidelines to avoid injury and decompression sickness.
Leash Laws in Georgia: Do Cats Need Them Too?
You may want to see also

Basketballs
When inflating a basketball, the pressure exerted by the gas inside is inversely proportional to the volume it occupies, as long as the temperature and the quantity of gas remain constant. This is the basis of Boyle's Law. The ideal gas law states that, for a closed system, the initial pressure divided by the initial volume is equal to the final pressure divided by the final volume. In other words, if the volume of air inside the basketball is increased, the pressure will decrease, and vice versa.
The NBA states that a basketball must be inflated to between 7.5 and 8.5 lb. of pressure. Using the ideal gas law, we can assume that a ball is filled at room temperature, and estimate that for every 10-degree change in temperature, the ball's pressure will increase or decrease by one pound. For example, a ball inflated to 8.5 lb. of pressure at 75 degrees Fahrenheit will drop to 7.37 lb. of pressure if the temperature is lowered to 65 degrees Fahrenheit.
The pressure inside a basketball is also important for how the ball bounces. When a basketball bounces, the volume inside the ball decreases and the pressure increases, and the increasing pressure springs the ball back up. The elasticity of the rubber also plays a role in the bounce, but the decrease in volume and increase in pressure is an application of Boyle's Law.
Boyle's Law was put forward by Anglo-Irish chemist Robert Boyle in 1662.
Understanding Scott's Law: Citizen Responsibilities and Legal Implications
You may want to see also

Syringes
A syringe is a medical device used to insert or withdraw fluids. It consists of a cylinder to hold the fluid and a plunger that can be pushed or pulled to change the pressure.
When the plunger is pushed down, the volume of the fluid inside the syringe decreases, and the pressure increases. Conversely, when the plunger is pulled up, the volume of the fluid increases, and the pressure decreases.
This is because gases are compressible—they will expand to fill any space that is available to them. When the plunger is pulled up, the volume of the gas increases, and the pressure decreases, creating a vacuum. This vacuum causes fluid to be sucked into the syringe through the needle. Conversely, when the plunger is pushed down, the volume of the gas decreases and the pressure increases, forcing fluid out of the syringe through the needle.
The operation of a syringe is a simple but perfect demonstration of Boyle's Law, which states that the volume of a gas is inversely proportional to its pressure, provided the temperature and the amount of gas remain constant.
Property Law Leases: Commercial Applications Explored
You may want to see also
Frequently asked questions
When you breathe in, your lung volume increases and the pressure within decreases, allowing air to be drawn into your lungs. The opposite happens when you breathe out.
As scuba divers go deeper underwater, the pressure increases and volume decreases, causing nitrogen gas to be absorbed by their blood. When they ascend, the pressure is lessened and the gas molecules begin to expand back to their normal volume. Divers must rise slowly to allow the gas to work its way back out of their bloodstream.
Spray paint cans contain two substances: the paint itself, and a gas that is pressurised so much that it retains a liquid state. When the nozzle is pushed down, the seal is broken and the gas boils, expands into a gas, and pushes the paint out of the nozzle.
When the plunger is pulled back, the interior volume of the syringe increases and its pressure reduces, creating a vacuum. The vacuum sucks fluid into the barrel until the interior and exterior pressure are balanced.







