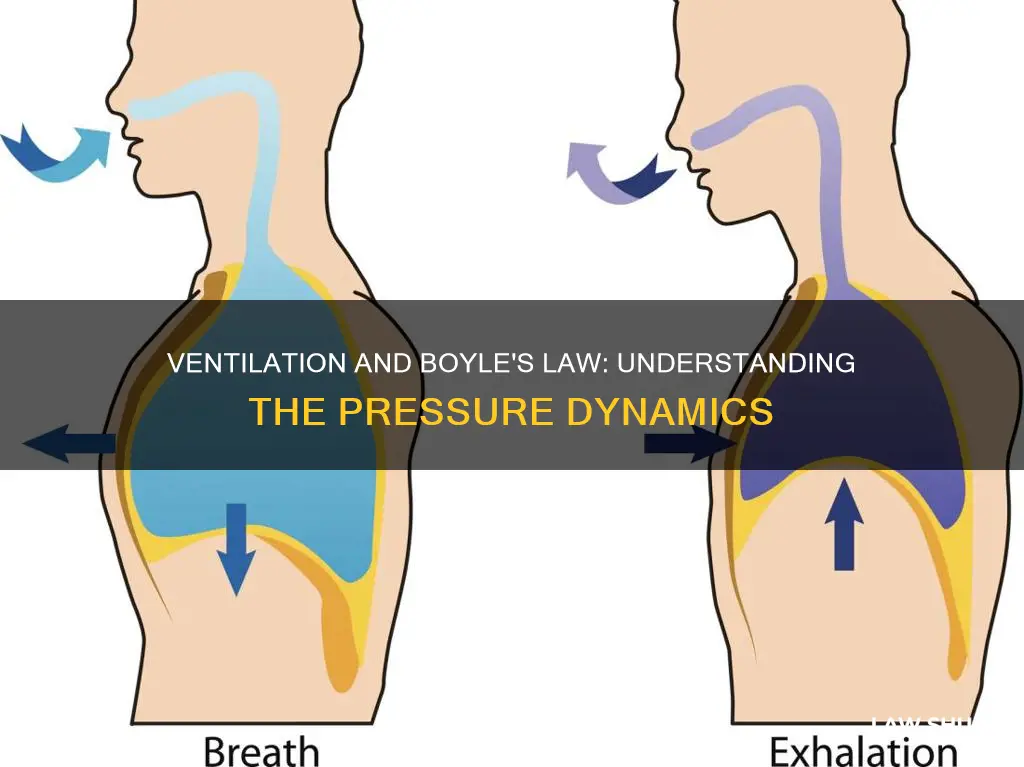
Boyle's Law, discovered by Robert Boyle in 1662, states that the volume of a gas and pressure are inversely proportional at a given temperature. This means that when the pressure increases, the volume decreases, and vice versa. This law is particularly important in understanding the mechanics of the human respiratory system. When we inhale, our diaphragm lowers, increasing the volume inside our lungs and decreasing the pressure, which causes air to be drawn into our lungs. Conversely, when we exhale, our diaphragm pushes upwards, reducing the volume inside our lungs, increasing the pressure, and forcing air outwards.
| Characteristics | Values |
|---|---|
| Relationship between pressure and volume of a gas | The volume of a gas and pressure are inversely proportional at a given temperature. |
| Application to breathing | When you inhale, your diaphragm lowers, increasing the volume inside your lungs and making the air pressure inside your lungs lower than the air pressure outside your body, drawing outside air into your lungs. |
| Application to syringes | When the plunger of a syringe is pulled out, the volume inside the barrel increases, resulting in a decrease in the pressure inside the barrel, drawing fluid into the syringe. |
| Application to SCUBA diving | As a diver descends into the water, the pressure on their lungs increases, so the air volume inside the lungs must decrease. As the diver ascends in the water and the pressure on the thoracic cage decreases, the volume of air increases. |
What You'll Learn

How does Boyle's Law apply to breathing?
Boyle's law states that the volume of a gas and pressure are inversely proportional at a given temperature. In other words, when the pressure increases, the volume decreases, and vice versa. This law is important because it helps us understand the relationship between pressure and volume in the lungs when breathing.
During inhalation, the diaphragm (a large muscle below the lungs) lowers, increasing the volume inside the lungs. This increase in volume leads to a decrease in air pressure inside the lungs, allowing outside air to be drawn into the lungs. This process follows Boyle's law, which dictates that as volume increases, pressure must decrease.
When exhaling, the diaphragm pushes upwards, reducing the volume inside the lungs and causing an increase in air pressure. This pressure increase forces the air out of the lungs. Again, this process aligns with Boyle's law, demonstrating the inverse relationship between volume and pressure.
The application of Boyle's law to breathing helps us understand the mechanics of respiration and the role of the diaphragm in maintaining the necessary pressure and volume changes for inhalation and exhalation. It also highlights the importance of maintaining a balance between volume and pressure within the lungs to ensure efficient gas exchange.
Furthermore, Boyle's law has clinical significance in respiratory conditions such as pneumothorax, where increased pressure within the intrapleural space affects the ability to generate negative pressure during inhalation, impacting the airflow into the lungs. Understanding Boyle's law is crucial for healthcare professionals when addressing respiratory issues and for divers to avoid pulmonary barotrauma when ascending and descending underwater.
Understanding Community Property Laws in Arizona
You may want to see also

How does Boyle's Law apply to syringes?
Boyle's Law, discovered by Robert Boyle in 1662, states that the volume of a given mass of gas is inversely proportional to the absolute pressure exerted on it, assuming the temperature remains constant. In other words, an increase in pressure leads to a decrease in volume, and vice versa. This principle has various applications, including in ventilation and the functioning of syringes.
Syringes operate based on Boyle's Law. When the plunger of a syringe is pulled out, the volume inside the barrel increases, leading to a decrease in pressure. According to Boyle's Law, this change in pressure results in fluids (such as water) flowing from areas of high pressure to the low-pressure area within the syringe. Therefore, when the pressure inside the syringe becomes lower than the pressure outside, fluids near the needle are drawn into the syringe. Conversely, when the plunger is pushed back in, reducing the volume and increasing the pressure inside the barrel, the fluid inside is forced out.
The relationship between pressure and volume described by Boyle's Law is also applicable to the respiratory system. During inhalation, the diaphragm lowers, increasing the volume inside the lungs and decreasing the air pressure within them. As a result, air from outside at a higher pressure rushes into the lungs. Conversely, when exhaling, the diaphragm pushes upwards, reducing the volume inside the lungs, which increases the pressure and forces air out.
Boyle's Law helps explain the behaviour of gases under varying conditions of temperature, pressure, and volume. It is one of the gas laws, along with Gay-Lussac's Law and Graham's Law, that collectively form the ideal gas law.
Sex Laws in China: Foreigners and Their Rights
You may want to see also

How does Boyle's Law apply to SCUBA divers?
Boyle's Law is incredibly important for SCUBA divers to understand, as it helps them anticipate how air will behave during a dive and explains many of the safety guidelines they must follow.
Boyle's Law states that the volume of a gas and pressure are inversely proportional at a given temperature. In other words, when the pressure increases, the volume decreases, and vice versa. This law only applies at a constant temperature; if the temperature of a gas changes, the equation no longer works.
When a diver descends, the water pressure around them increases, causing the air in their scuba equipment and body to compress and occupy a smaller volume. Conversely, as a diver ascends, water pressure decreases, and the air in their gear and body expands to occupy a greater volume. This is why divers must release excess air from their buoyancy compensator device (BCD) as they ascend, or they will lose control of their buoyancy.
Many of the safety protocols in scuba diving are designed to help divers compensate for the compression and expansion of air due to changes in water pressure. For example, the compression and expansion of gas lead to the need to equalise your ears, adjust your BCD, and make safety stops.
One of the most important safety rules derived from Boyle's Law is to never hold your breath underwater. This is because, if a diver ascends while holding their breath, the air trapped in their lungs will expand according to Boyle's Law and can cause pulmonary barotrauma. Divers are also told to ascend slowly. This is because, as a diver ascends, the compressed nitrogen gas in their body expands. If they do not ascend slowly enough for their body to eliminate this expanding gas, it can form tiny bubbles in their blood and tissue and cause decompression sickness.
Employment Laws: Who Is Covered and Who Isn't?
You may want to see also

How does Boyle's Law apply to weather balloons?
Weather balloons are a prime example of Boyle's Law in action. These balloons are sent to high altitudes, carrying instruments to measure atmospheric variables such as pressure, temperature, and wind speed. Interestingly, weather balloons are only partially filled with gas, usually helium, before they are launched. This is because, as they ascend, the external air pressure decreases, causing the volume of gas inside the balloon to increase due to Boyle's Law. If the balloon were filled to its maximum capacity at the higher pressure at the Earth's surface, the subsequent increase in volume at lower pressures in the upper atmosphere would cause the balloon to burst.
Boyle's Law, discovered by Robert Boyle in 1662, states that the volume of a gas is inversely proportional to the pressure exerted by the gas, provided the temperature remains constant. This law can be expressed mathematically as pV=k, where p is the pressure of the gas, V is the volume of the gas, and k is a constant.
In the context of a weather balloon, as the balloon rises, the pressure exerted by the helium inside the balloon remains constant, but the external pressure decreases. According to Boyle's Law, this decrease in external pressure results in an increase in the volume of helium inside the balloon. Therefore, the balloon expands as it ascends to higher altitudes with lower air pressure.
The application of Boyle's Law to weather balloons is a critical consideration in their design and operation. By understanding this principle, scientists and engineers can ensure that the balloons are filled to an appropriate level, taking into account the expected change in volume as the balloon ascends. This knowledge helps prevent the balloons from bursting due to excessive expansion, allowing them to safely carry out their important role in atmospheric measurements.
Exploring Space's Legal Boundaries with Maritime Law
You may want to see also

How does Boyle's Law apply to lung compliance?
Boyle's law states that the volume of a gas and pressure are inversely proportional at a given temperature. In other words, when the pressure increases, the volume decreases, and vice versa. This law is important because it helps us understand the relationship between pressure and volume in the lungs when breathing.
The lungs do not follow Boyle's law at all volumes. When the alveoli are not collapsed and the lungs are not at maximum capacity, the lungs follow proportional changes in volume and pressure, as per Boyle's law. At low lung volumes, it takes a large change in pressure to make small changes in volume (low compliance of lung tissue). Conversely, at high volumes within the lung, it takes more negative pressure to expand the tissue, indicating a decrease in tissue elasticity. This is also considered low compliance. Compliance is calculated using the formula: compliance = [change in volume]/[change in pressure].
During inhalation, the diaphragm (a large muscle below the lungs) lowers, increasing the volume inside the lungs. This makes the air pressure inside the lungs lower than the air pressure outside the lungs and body, and outside air is drawn into the lungs. When you exhale, the diaphragm pushes upwards, reducing the volume inside the lungs, increasing the pressure, and forcing the air outwards.
Demorgan's Law: Applicable to What?
You may want to see also