Coulomb's Law, an experimental law of physics, calculates the amount of force between two electrically charged particles at rest. It states that the magnitude of the attractive or repulsive force between two charges is directly proportional to the product of their charges and inversely proportional to the square of the distance between them. The law was first published in 1785 by French physicist Charles-Augustin de Coulomb, who mathematically described the force between charged objects using careful measurements of forces between charged spheres. Coulomb's Law is an inverse-square law, similar to Isaac Newton's inverse-square law of universal gravitation, but with some key differences. Coulomb's Law is applicable to both attractive and repulsive forces, depending on the charges involved. It is an important concept in physics and has wide-ranging applications.
What You'll Learn

Coulomb's Law is an inverse-square law
The law can be expressed as:
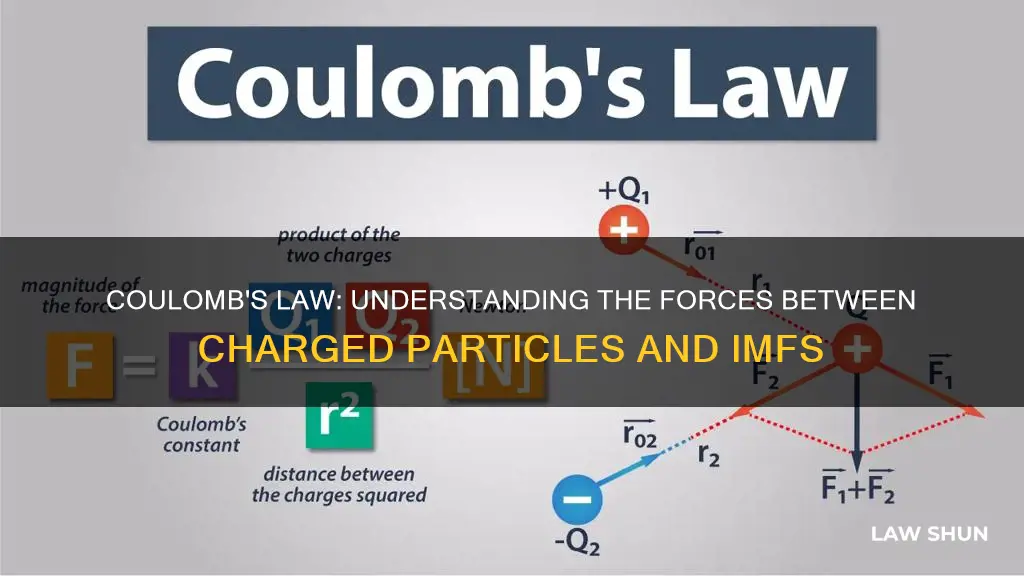
|F| = ke * (q1 * q2) / r^2
Where ke is a constant, q1 and q2 are the quantities of each charge, and r is the distance between the charges.
Coulomb's Law is similar to Isaac Newton's inverse-square law of universal gravitation. However, gravitational forces always cause attraction, while electrostatic forces can cause charges to attract or repel. Gravitational forces are also much weaker than electrostatic forces.
Coulomb's Law is an experimental law of physics and was first published in 1785 by French physicist Charles-Augustin de Coulomb. It was essential to the development of the theory of electromagnetism. The law has been tested extensively and has been upheld on a scale from 10^-16 m to 10^8 m.
Antique Firearms: Concealed Carry Law Exemptions?
You may want to see also

The electrostatic force is called the Coulomb force
Coulomb's law states that the magnitude of the attractive or repulsive electrostatic force between two point charges is directly proportional to the product of the magnitudes of their charges and inversely proportional to the square of the distance between them.
The force is along the straight line joining the two charges. If the charges have the same sign, the electrostatic force between them makes them repel; if they have different signs, the force between them makes them attract.
Coulomb's law is an experimental law of physics that calculates the amount of force between two electrically charged particles at rest. The law is similar to Isaac Newton's inverse-square law of universal gravitation, but gravitational forces always make things attract, while electrostatic forces make charges attract or repel.
Coulomb's law can be used to calculate the force between two charges, the distance between two charges, and to arrange the charges in stable equilibrium. It also helps determine the force acting at a point due to various charges.
Usury Laws and Personal Loans: What's the Verdict?
You may want to see also

The law was first published in 1785 by Charles-Augustin de Coulomb
Charles-Augustin de Coulomb, a French mathematician and physicist, first published Coulomb's Law in 1785. Coulomb's Law is an experimental law of physics that calculates the amount of force between two electrically charged particles at rest.
Coulomb's Law states that the magnitude, or absolute value, of the attractive or repulsive electrostatic force between two point charges is directly proportional to the product of the magnitudes of their charges and inversely proportional to the square of the distance between them. In other words, the force between two charged particles is directly proportional to the product of their charges and inversely proportional to the square of the distance between them.
Coulomb discovered that bodies with like electrical charges repel each other, while oppositely charged bodies attract. Coulomb's Law can be used to describe the attraction and repulsion between any charged particles, including atomic particles. It holds even within atoms, correctly describing the force between the positively charged atomic nucleus and each of the negatively charged electrons.
Coulomb's Law is defined as:
F = Force of attraction or repulsion between the charges Q1, Q2 = Magnitude of charge 1 and charge 2
D = Distance between two charges
K = Constant whose value depends on the medium in which the charges are placed
Ε0 = permittivity of vacuum
Εr = relative permittivity of the medium with respect to free space
Coulomb's Law was essential to the development of the theory of electromagnetism and was perhaps even its starting point. It allowed meaningful discussions of the amount of electric charge in a particle. The law also underlies much of atomic physics.
Yale or Yale Law: Which Path to Take?
You may want to see also

The law is only applicable to point charges
Coulomb's law is a mathematical concept that defines the electric force between charged objects. It states that the force between any two charged particles is directly proportional to the product of their charges and inversely proportional to the square of the distance between them. The law is applicable to point charges that are smaller than the distance between them. When the size of charged bodies is substantially smaller than the separation between them, the size is not considered or can be ignored, and the charged bodies can be considered point charges.
Coulomb's law is defined for point charges. For large bodies, one needs to integrate the partial forces exerted on differential volumes of the bodies. However, for uniformly charged spheres or spherical shells, Coulomb's law can be used by inserting the distance between the centres of the spheres in the formula. This can be proved by integration, but it is difficult. Coulomb's law does not apply to two charged bodies of finite sizes, as the distribution of charge does not remain uniform when the two bodies are brought together.
Coulomb's law is valid for distributions, but the integral form must be used. The equation refers to the distance from the source, which is only defined for a point, not a distribution. Coulomb's law is also not valid for moving charges because the information about the position of the charge (the field caused by the charge) can only travel at the speed of light.
Case Law Across Borders: Scotland and England
You may want to see also

The law can be used to calculate the force between two charges
Coulomb's Law, or Coulomb's inverse-square law, is a mathematical formula that defines the electric force between two electrically charged particles at rest. It is expressed as:
F = k * (q1 * q2) / r^2
Where:
- F is the force between the charges
- K is Coulomb's constant (approximately 8.9875 x 10^9 Nm^2/C^2)
- Q1 and q2 are the magnitudes of the charges
- R is the shortest distance between the charges
The force acts along the line joining the two charges. If the charges have the same sign, the force is repulsive; if they have different signs, the force is attractive.
To calculate the force between two charges using Coulomb's Law, follow these steps:
- Find the charges q1 and q2 in coulombs.
- Multiply the charges together.
- Multiply the result by the constant k.
- Divide the result by the square of the distance between the charges.
- The final result is the force between the charges. If the force is positive, it is repulsive; if it is negative, it is attractive.
Thermodynamics Laws: Universal or Not?
You may want to see also
Frequently asked questions
Coulomb's Law is a fundamental principle in physics that defines the electric force between charged objects. It states that the magnitude of the force between two charged particles is directly proportional to the product of their charges and inversely proportional to the square of the distance between them.
No, Coulomb's Law can also be applied to non-point charges, such as charged spheres or cylinders. However, the charges must be concentrated in a small enough area to be treated as point charges for the equation to be accurate.
Yes, Coulomb's Law applies to both attractive and repulsive forces. The direction of the force depends on the sign of the charges. Like charges (positive-positive or negative-negative) will repel each other, while opposite charges (positive-negative) will attract.
Coulomb's Law and Newton's Law are similar in their inverse-square nature and the roles of mass and charge. However, they differ in that Newton's Law deals with gravitational force between masses, while Coulomb's Law deals with electrostatic forces between charges.
The constant, also known as the Coulomb constant, has the units of Newton-meter squared per coulomb squared (N•m2/C2). This ensures the equation has the correct units: Force (N) = Coulomb constant x (product of charges in coulombs) / (distance in meters squared).







