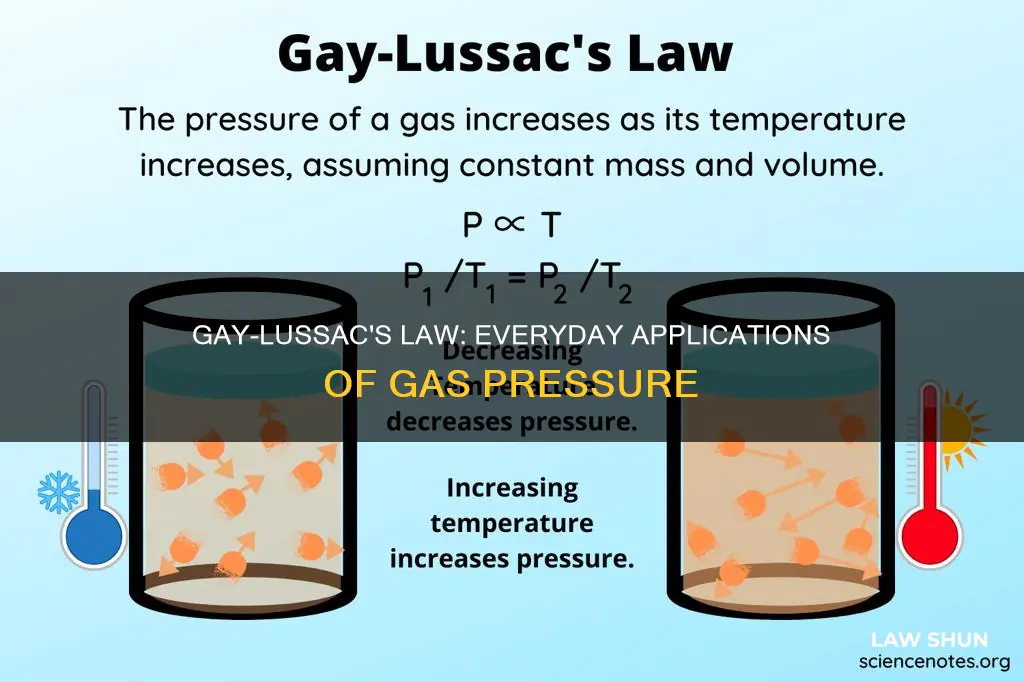
Gay-Lussac's Law, formulated by French chemist Joseph Gay-Lussac in 1802, states that the pressure exerted by a gas is directly proportional to its temperature when its volume and mass are kept constant. This law has several real-life applications, and understanding it is crucial for making the products we use daily, such as refrigerators and air conditioners. For instance, it explains why pressurised aerosol containers must be kept away from fire and why car tires are more prone to explosions during hot summer months.
| Characteristics | Values |
|---|---|
| Tire pressure | Drops on a cold day and soars on a hot day |
| Pressure cooker | Applying heat increases the pressure inside the cooker, shortening cooking times |
| Aerosol can | Heating the can increases the pressure of its contents, potentially causing it to burst |
| Water heater | Similar to a pressure cooker, with a pressure-relief valve to prevent steam from accumulating |
| Firing a bullet | The burning of gunpowder creates superheated gas, the high pressure of which forces the bullet out of the barrel of the gun |
| Car tires | The air pressure in a car's tires increases after driving due to friction between the tires and the road |
| Soda can | A cold can of soda fizzes less when opened, while a warm can fizzes more |
What You'll Learn

Tire pressure changes with temperature
Gay-Lussac's Law, formulated by French chemist Joseph Gay-Lussac in 1802, states that the pressure exerted by a gas of a given mass kept at a constant volume varies directly with the absolute temperature of the gas. In other words, the pressure exerted by a gas is proportional to its temperature when its mass is fixed and its volume is constant. This law can be observed in everyday life through examples such as aerosol cans, pressure cookers, and automobile tires.
Gay-Lussac's Law is evident in the fluctuation of automobile tire pressure with changes in temperature. As the seasons change and temperatures fluctuate, the pressure inside car tires will vary, leading to potential overinflation or underinflation. When air molecules inside the tires are heated, they expand, leading to increased tire pressure. Conversely, when temperatures drop, the air molecules in the tires condense, resulting in a loss of pressure.
This relationship between temperature and tire pressure can be explained by Gay-Lussac's Law. As the temperature inside the tires increases, the kinetic energy of the gas molecules also increases, causing them to move faster and collide more frequently with the walls of the tires. These increased collisions result in higher pressure. Similarly, when the temperature decreases, the gas molecules move slower and collide less frequently, reducing the pressure.
To maintain proper tire pressure, it is essential to monitor it regularly, especially during seasonal changes. For every 10°F fluctuation in ambient temperature, vehicle tire pressure can change by about 1 psi. Therefore, a seasonal shift from summer to winter can cause a pressure drop of more than 4 psi. Ensuring optimal tire pressure is crucial for safety, fuel efficiency, and preventing premature wear on the tires.
In summary, Gay-Lussac's Law explains how temperature fluctuations directly impact the pressure inside car tires. By understanding this law, drivers can take proactive measures to maintain proper tire pressure, ensuring a smoother ride and safer driving experience.
Open Meetings Law: Does It Apply in Kansas?
You may want to see also

Pressure cookers use Gay-Lussac's Law to cook food faster
Gay-Lussac's Law, formulated by French chemist Joseph Gay-Lussac in 1802, states that the pressure exerted by a gas of a given mass kept at a constant volume varies directly with its absolute temperature. In other words, the pressure exerted by a gas is proportional to its temperature when its mass is fixed and its volume is constant. This law has several applications in everyday life, one of which is in pressure cookers.
The increase in pressure inside the cooker raises the boiling point of water. This is why food cooks faster in a pressure cooker. Additionally, because the container is sealed, flavours are not lost to the air with steam. Tough meat also becomes much more tender when cooked in a pressure cooker due to the high temperature and pressure.
Gay-Lussac's Law can also be observed in other everyday scenarios, such as in car tyres. The air pressure inside car tyres increases after driving due to friction between the tyres and the road, which heats up the air inside. Since the tyres are essentially a fixed-volume container, the air cannot expand, so the pressure increases, in accordance with Gay-Lussac's Law.
Humanitarian Law and Hamas: A Complex Legal Question
You may want to see also

Exploding aerosol cans
Gay-Lussac's Law states that the pressure exerted by a gas of a given mass and kept at a constant volume varies directly with the absolute temperature of the gas. In other words, the pressure exerted by a gas is proportional to the temperature of the gas when the mass is fixed and the volume is constant. This law was formulated by French chemist Joseph Gay-Lussac in 1802 (or 1808, according to one source).
The consequences of an exploding aerosol can be severe, especially if the can contains flammable liquids or gases. A huge fireball can be created, as the unfortunate camper in the story Gay-Lussac read in the newspaper found out the hard way.
To avoid explosions, it is important to store aerosol cans in cool, shaded places and never expose them to high temperatures or open flames.
Security Deposit Laws: Sublease Rights and Responsibilities
You may want to see also

Firing a bullet
Gay-Lussac's Law, formulated by French chemist Joseph Gay-Lussac in 1802, states that the pressure exerted by a gas is directly proportional to its temperature when its mass is fixed and its volume is constant. Mathematically, this relationship can be expressed as:
> P ∝ T
>
> P1/T1 = P2/T2
>
> P1T2 = P2T1
Where:
- P is the pressure exerted by the gas
- T is the absolute temperature of the gas
In the context of firing a bullet, the burning gunpowder rapidly increases the temperature and pressure of the gas inside the gun barrel. This increase in pressure exerts a force on the bullet, causing it to accelerate down the barrel and exit at high speed. The principle is similar to that of a pressure cooker, where heating the contents increases the pressure, allowing food to cook faster.
Gay-Lussac's Law also has other everyday applications, such as in aerosol cans, where heating the can increases the internal pressure, potentially leading to an explosion. It is important to understand and apply Gay-Lussac's Law in various situations to ensure safety and optimize performance, whether it's in the context of firearms, automotive tires, or even kitchen appliances.
California Landlord-Tenant Law: Commercial Property Rules Explained
You may want to see also

Soda cans fizz more when warm
Gay-Lussac's Law, formulated by French chemist Joseph Gay-Lussac in 1802, states that the pressure exerted by a gas of a given mass kept at a constant volume varies directly with the absolute temperature of the gas. In simpler terms, the pressure exerted by a gas is proportional to its temperature when its mass is fixed and its volume is constant. This law has several applications in everyday life, and one such instance is seen in soda cans.
Additionally, when the temperature rises, the gases inside the soda can expand, creating higher pressure. This increased pressure further contributes to the loss of carbonation by pushing the carbon dioxide out of the liquid. The combination of these factors results in a more pronounced fizzing or bubbling effect when a warm soda can is opened compared to a cold one.
To maintain the desired level of carbonation in soda cans, it is essential to store them in cool environments and avoid exposure to high temperatures. This helps to slow down the escape of carbon dioxide from the liquid, preserving the fizziness of the beverage.
Gay-Lussac's Law also has other practical applications in everyday life. For example, it explains why automobile tire pressure increases on hot days and decreases on cold days. Similarly, it is relevant to the functioning of pressure cookers, where heating the contents increases the pressure inside, leading to faster cooking times. Understanding Gay-Lussac's Law helps us make sense of various phenomena involving gases and pressure changes in our daily lives.
Gift Tax and Your Child-in-Law: What You Need to Know
You may want to see also
Frequently asked questions
Gay-Lussac's Law explains why car tires explode more during hot summer months. As the temperature of the gas inside the tire increases, so does the pressure, which can exceed the tire's elastic capabilities.
Pressure cookers work by increasing the temperature and pressure of steam inside a sealed container. This raises the boiling point of water, cooking food faster and making tough meat more tender.
Heating an aerosol can increases the pressure of its contents, which is why they should not be stored in hot conditions or disposed of by burning. The increased pressure can cause the can to burst.
The pressure gauge on a propane tank will read higher when the temperature is higher, and lower when the temperature is lower. This is because the pressure of the gas in the tank is directly proportional to its temperature.
A cold soda will fizz less when opened compared to a warm soda, which will fizz more. This is because the pressure inside the can is higher when the can and its contents are warmer.







