Glycolysis is a biological process that breaks down glucose to produce energy. The first law of thermodynamics states that energy can neither be created nor destroyed, only converted from one form to another. This law is highly relevant to glycolysis as it underpins the entire process. During glycolysis, the potential chemical energy in glucose is converted into other forms of energy, such as ATP and heat. This process is essential for life, as it allows cells to maintain their organisation and perform work, such as metabolism and membrane transport.
| Characteristics | Values |
|---|---|
| First Law of Thermodynamics | Energy can be converted from one form to another, but never created or destroyed |
| Application to Glycolysis | In glycolysis, glucose (potential chemical energy) is converted into ATP (another form of potential chemical energy) and heat (radiant energy) |
What You'll Learn
- The first law of thermodynamics states that energy can be converted from one form to another but never created or destroyed
- In glycolysis, glucose (potential chemical energy) is converted into ATP (another form of potential chemical energy) and heat (radiant energy)
- Glycolysis is a sequence of reactions leading from one intermediate compound to the next, with energy changes associated with each reaction
- The first law of thermodynamics implies that the total amount of energy in a closed system remains constant
- The first law of thermodynamics is intimately linked to the release of energy in biological systems

The first law of thermodynamics states that energy can be converted from one form to another but never created or destroyed
This law has important implications for biological systems, which are dependent on the release of energy for their functioning. For example, during photosynthesis, plants convert solar (radiant) energy into chemical energy. Similarly, during respiration, mitochondria convert glucose (potential chemical energy) into ATP (another form of potential chemical energy) and heat (radiant energy). This process involves a flow of electrons through a series of electron carriers, demonstrating the conversion of electrical energy.
Glycolysis, the process of breaking down glucose, is a sequence of reactions that lead from one intermediate compound to the next. Each of these reactions is associated with energy changes, some of which may be endothermic and others exothermic. By mapping out these energy changes, we can see the overall energy profile of glycolysis, which may resemble a roller coaster ride with energetic drops and hills.
However, the relative concentrations of the different species under cellular conditions need to be considered to fully understand the energetic landscape of glycolysis. This leads to a slightly different picture, where glycolysis exists mostly on an energetic plain, with only a few steep drops.
The first law of thermodynamics highlights the importance of energy conservation and the ability to convert energy between different forms. In biological systems, this law underpins the processes that enable organisms to maintain their organization and carry out essential life functions.
Phone Laws: Exempting Tesla Drivers?
You may want to see also

In glycolysis, glucose (potential chemical energy) is converted into ATP (another form of potential chemical energy) and heat (radiant energy)
The first law of thermodynamics states that energy can be converted from one form to another but never created or destroyed. In glycolysis, glucose (potential chemical energy) is converted into ATP (another form of potential chemical energy) and heat (radiant energy). This process is an example of the first law of thermodynamics in action, as energy is transformed from one form to another without any creation or destruction.
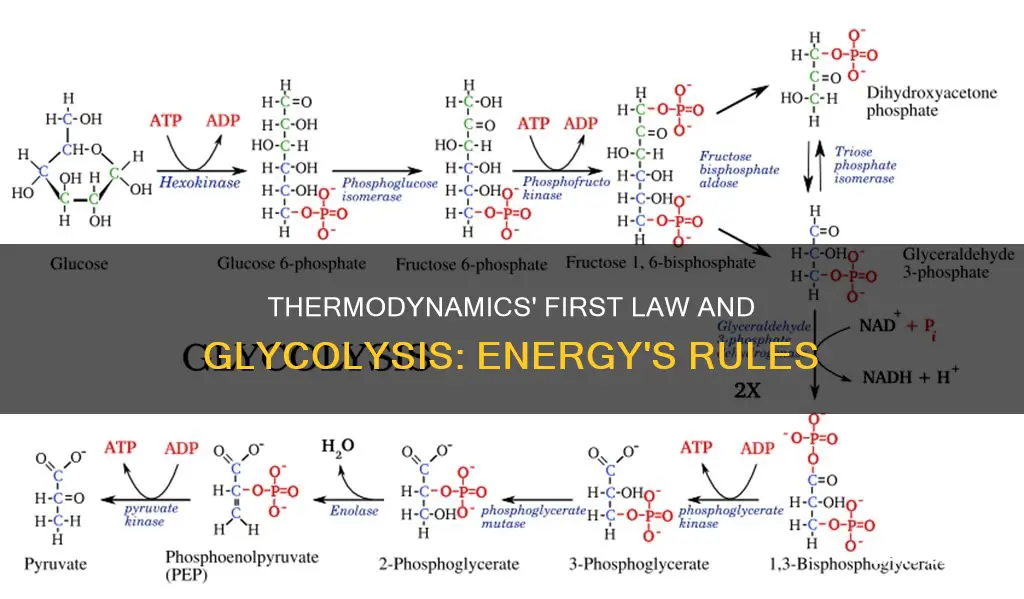
Glycolysis is a metabolic pathway that occurs in the liquid part of cells, known as the cytosol. It involves a sequence of ten reactions, each catalysed by a specific enzyme. The process can be divided into two phases: the investment phase, where ATP is consumed, and the yield phase, where more ATP is produced than initially used.
The first step of glycolysis is the phosphorylation of glucose by the enzyme hexokinase, forming glucose 6-phosphate (G6P). This step is crucial as it keeps the glucose concentration inside the cell low, promoting the continuous transport of blood glucose into the cell. Additionally, phosphorylation prevents the glucose from leaking out, as the cell lacks transporters for G6P.
The second step involves rearranging G6P into fructose 6-phosphate (F6P) by the enzyme phosphoglucose isomerase. Fructose can also enter the glycolytic pathway by phosphorylation at this stage. This isomerization step is freely reversible under typical cellular conditions but is often driven forward due to the low concentration of F6P, which is rapidly consumed in the subsequent step.
The third step is the phosphorylation of F6P, forming fructose 1,6-bisphosphate (F1,6BP). This step is energetically favourable and justifies the energy expenditure of another ATP molecule. It also ensures the formation of two charged groups, preventing the free diffusion of substrates out of the cell.
In the fourth step, aldolase cleaves the hexose ring of F1,6BP, forming two triose sugars: dihydroxyacetone phosphate (a ketose) and glyceraldehyde 3-phosphate (an aldose). The electrons delocalized in the carbon-carbon bond cleavage associate with the alcohol group, and the resulting carbanion is stabilised by the structure of the carbanion itself and the presence of a charged ion prosthetic group.
The fifth step involves the rapid interconversion of dihydroxyacetone phosphate and glyceraldehyde 3-phosphate by the enzyme triosephosphate isomerase. This step simplifies regulation by directing both triose sugars down the same pathway.
The second half of glycolysis, known as the pay-off phase, is characterised by a net gain of energy-rich molecules ATP and NADH. The aldehyde groups of the triose sugars are oxidised, and inorganic phosphate is added, forming 1,3-bisphosphoglycerate. The hydrogen from this step is used to reduce NAD+ to NADH for each triose sugar molecule.
The seventh step is the enzymatic transfer of a phosphate group from 1,3-bisphosphoglycerate to ADP by phosphoglycerate kinase, forming ATP and 3-phosphoglycerate. This step is crucial as it represents the break-even point in the glycolytic pathway, where the consumption and production of ATP are equal.
In the eighth step, phosphoglycerate mutase isomerises 3-phosphoglycerate into 2-phosphoglycerate. The ninth step involves the conversion of 2-phosphoglycerate to phosphoenolpyruvate by the enzyme enolase.
The final step of glycolysis is catalysed by the enzyme pyruvate kinase, forming pyruvate and another ATP molecule. This step serves as an additional regulatory point in the pathway, similar to the phosphoglycerate kinase step.
Overall, glycolysis can be summarised by the following equation:
> Glucose + 2 NAD+ + 2 ADP + 2 Pi → 2 Pyruvate + 2 NADH + 2 H+ + 2 ATP + 2 H2O
In this process, glucose (potential chemical energy) is converted into pyruvate, ATP (another form of potential chemical energy), and heat (radiant energy). This conversion of energy from one form to another aligns with the first law of thermodynamics, highlighting the fundamental role of this principle in biological systems.
Oregon's Real Estate Disclosure Law: Commercial Property Exemption
You may want to see also

Glycolysis is a sequence of reactions leading from one intermediate compound to the next, with energy changes associated with each reaction
Glycolysis is a sequence of reactions that convert glucose into pyruvate. The process can be divided into two phases: the energy investment phase and the energy payout phase.
The energy investment phase involves two reactions catalysed by the enzymes hexokinase and phosphofructokinase. In the first reaction, hexokinase catalyses the phosphorylation of glucose to form glucose-6-phosphate (G6P), consuming one molecule of adenosine triphosphate (ATP). This reaction is spontaneous and irreversible, trapping the glucose molecule inside the cell as G6P cannot pass through the cell membrane. In the second reaction, phosphofructokinase catalyses the phosphorylation of fructose-6-phosphate to form fructose-1,6-bisphosphate, consuming a second molecule of ATP. This step is also irreversible and is a key regulatory point in glycolysis.
The energy payout phase involves eight reactions, each leading from one intermediate compound to the next. Fructose-1,6-bisphosphate is converted into two triose sugars, glyceraldehyde-3-phosphate (GA3P) and dihydroxyacetone phosphate (DHAP), by the enzyme fructose-bisphosphate aldolase. DHAP is then converted into a second molecule of GA3P by the enzyme triosephosphate isomerase. Both molecules of GA3P then enter the payout phase, where a molecule of nicotinamide adenine dinucleotide (NADH) and two molecules of ATP are produced for each molecule of GA3P. The remaining reactions in the payout phase are catalysed by the enzymes glyceraldehyde phosphate dehydrogenase, phosphoglycerate kinase, phosphoglycerate mutase, enolase, and pyruvate kinase. Overall, the net result of glycolysis is the production of two molecules each of ATP, NADH, pyruvate, and water.
Hawaii's TVU Law: Does It Reach Neighboring Islands?
You may want to see also

The first law of thermodynamics implies that the total amount of energy in a closed system remains constant
The first law of thermodynamics states that energy can be converted from one form to another, but it cannot be created or destroyed. This means that the total amount of energy in a closed system, such as the universe, remains constant. In other words, energy cannot enter or leave a closed system.
This law has important implications for biological systems, including glycolysis. Glycolysis is a series of reactions that convert glucose (potential chemical energy) into ATP (another form of potential chemical energy) and heat (radiant energy). This process involves the flow of electrons through a series of electron carriers, converting energy from one form to another.
The first law of thermodynamics implies that the total amount of energy in a closed biological system remains constant. In glycolysis, the total amount of energy present at the beginning of the process is conserved throughout the series of reactions, even as the energy changes form. This is because energy is neither created nor destroyed during glycolysis; it is simply converted from one form to another.
For example, during glycolysis, glucose is split into two pyruvic acid molecules. This reaction involves the activation of glucose, requiring an input of energy, and the production of four ATP molecules, which store energy. The net yield of this process is two ATP molecules, as two ATP molecules were used to activate the glucose. Therefore, the total amount of energy in the system remains constant, in accordance with the first law of thermodynamics.
It is important to note that the first law of thermodynamics does not consider the efficiency of energy conversions. While energy may be conserved, the conversions may not be 100% efficient, with some energy being lost as heat or other forms of energy. This is addressed in the second law of thermodynamics, which states that the amount of available energy in a closed system is constantly decreasing due to entropy, or the increase in disorder or randomness of a system.
Truancy Laws: Do They Apply to 17-Year-Olds?
You may want to see also

The first law of thermodynamics is intimately linked to the release of energy in biological systems
The first law of thermodynamics is a formulation of the law of conservation of energy. It states that energy can be transferred and transformed but never created or destroyed. This law is intimately linked to the release of energy in biological systems.
Biological systems are constantly performing work, such as metabolism, membrane transport, and movement. To do this, they require energy. The first law of thermodynamics dictates that the energy used by biological systems must be transferred from or transformed from other forms of energy.
One of the most important energy transformations in biology is the conversion of radiant energy from the sun into chemical energy by plants. This is achieved through photosynthesis. The radiant energy is converted into chemical energy, which is stored within organic molecules such as sugars and fats. This stored chemical energy can then be transformed through a series of cellular chemical reactions into energy within molecules of ATP.
ATP molecules provide easily accessible energy for cells to perform work. This includes building complex molecules, transporting materials, powering the beating motion of cilia or flagella, contracting muscle fibers to create movement, and reproduction.
The first law of thermodynamics also applies to the breakdown of glucose during cellular respiration. During this process, 40% of the potential energy in glucose is converted to ATP, while the remaining 60% is converted to thermal and unusable energy.
Lemon Law and Private Sales: What's the Deal?
You may want to see also
Frequently asked questions
The first law of thermodynamics states that energy can be converted from one form to another, but it cannot be created or destroyed. The total amount of energy in a closed system remains constant.
Glycolysis is a series of reactions that convert glucose (potential chemical energy) into pyruvate and ATP (another form of potential chemical energy). This process involves the conversion of energy from one form to another, in line with the first law of thermodynamics.
Glycolysis is a crucial step in energy production, particularly in cellular respiration. It is the first step of glucose breakdown and is followed by fermentation or cellular respiration, depending on the availability of oxygen. This process ultimately leads to the release of energy that is harnessed by biological systems to carry out essential life functions.







