The Frank-Starling Law, also known as Starling's Law, describes the relationship between stroke volume and end-diastolic volume. The law states that the stroke volume of the heart increases in response to an increase in the volume of blood in the ventricles, before contraction (the end-diastolic volume), when all other factors remain constant. The Frank-Starling mechanism allows the cardiac output to be synchronized with the venous return, arterial blood supply, and humoral length, without depending on external regulation to make alterations. The mechanism is of functional importance because it serves to adapt left ventricular output to right ventricular output.
What You'll Learn
- The Frank-Starling law and the length-tension relationship in striated muscle
- The Frank-Starling law and the role of sarcomeres
- The Frank-Starling law and the function of the left ventricle
- The Frank-Starling law and the role of calcium sensitivity
- The Frank-Starling law and the impact on cardiac output

The Frank-Starling law and the length-tension relationship in striated muscle
The Frank-Starling law, also known as Starling's law or the Frank-Starling mechanism, describes the relationship between stroke volume and end-diastolic volume. The law states that the stroke volume of the heart increases in response to an increase in the volume of blood in the ventricles before contraction (end diastolic volume), assuming other factors remain constant. This mechanism allows the heart to automatically adjust its output to changes in venous return without the need for external regulation.
The Frank-Starling law is based on the length-tension relationship observed in striated muscle, including skeletal, arthropod, and cardiac muscle. As a muscle is stretched, the overlap of thick and thin filaments within the muscle changes, resulting in the development of active tension. In the case of the heart, this increase in tension leads to a stronger contraction, allowing the heart to pump out more blood.
The optimal length for contraction varies depending on the type of muscle. In skeletal muscle, the sarcomere length is typically maintained near optimal due to the fixed distance between attachment points. In contrast, the relaxed sarcomere length of cardiac muscle cells is usually shorter than optimal, and it can vary depending on blood filling and the expansion of the heart chambers.
The Frank-Starling mechanism has important clinical significance, particularly in the context of systolic heart failure. It helps compensate for the reduced cardiac output by increasing the stretch of myocardial fibers, leading to a greater stroke volume and preserving cardiac output. However, the benefit of this mechanism is limited, and severe heart failure can result in pulmonary congestion.
The Frank-Starling law also plays a role in patients with dilated cardiomyopathy, where both the right and left ventricles are dilated and exhibit decreased contractile function. The increased ventricular diastolic volume stretches the myocardial fibers, resulting in a subsequent increase in stroke volume. Additionally, neurohormonal activation mediated by the sympathetic nervous system compensates for the reduced cardiac output by increasing heart rate and contractility.
Interpreting Laws: Power and Application
You may want to see also

The Frank-Starling law and the role of sarcomeres
The Frank-Starling law describes the relationship between the force or tension developed in a muscle fibre and the extent to which the fibre is stretched. In the context of the heart, this means that the greater the stretch of the ventricle in diastole, the greater the stroke volume achieved in systole. This is due to the link between the initial length of myocardial fibres and the force generated by contraction.
Sarcomeres are the basic contractile units of a muscle fibre. The Frank-Starling law is based on the observation that there is a predictable relationship between the length between sarcomeres and the tension of the muscle fibres. There is an optimal length between sarcomeres at which the tension in the muscle fibre is greatest, resulting in the greatest force of contraction. If sarcomeres are closer together or further apart compared to this optimal length, there will be a decrease in contraction tension and strength.
The greater the ventricular diastolic volume, the more the myocardial fibres are stretched during diastole. Within a normal physiological range, the more the myocardial fibres are stretched, the greater the tension in the muscle fibres and the greater the force of contraction of the ventricle when stimulated. This is because the Frank-Starling law also describes the interrelationship between end-diastolic volume and cardiac ejection volume, a regulatory system that operates on a beat-to-beat basis.
The main cellular mechanism underlying this phenomenon is an increase in the responsiveness of cardiac myofilaments to activating Ca2+ ions at a longer sarcomere length, commonly referred to as myofilament length-dependent activation. However, it is still not known exactly how the contractile apparatus transduces the information concerning sarcomere length to modulate ventricular pressure development.
Copyright Law: Film's Friend or Foe?
You may want to see also

The Frank-Starling law and the function of the left ventricle
The Frank-Starling law, also known as Starling's law and the Frank-Starling mechanism, describes the relationship between stroke volume and end-diastolic volume. The law states that the stroke volume of the heart increases in response to an increase in the volume of blood in the ventricles before contraction (the end-diastolic volume), assuming all other factors remain constant. This mechanism allows the cardiac output to be synchronized with the venous return, arterial blood supply, and humoral length, without depending on external regulation.
The Frank-Starling mechanism is the result of the length-tension relationship observed in striated muscle, including skeletal muscles, arthropod muscle, and cardiac muscle. As striated muscle is stretched, active tension is created by altering the overlap of thick and thin filaments. The greatest isometric active tension is developed when a muscle is at its optimal length. In most relaxed skeletal muscle fibres, the passive elastic properties maintain the muscle fibres' length near optimal, as determined by the fixed distance between the attachment points of tendons to the bones at either end of the muscle.
In contrast, the relaxed sarcomere length of cardiac muscle cells in a resting ventricle is lower than the optimal length for contraction. There is no bone to fix sarcomere length in the heart, so sarcomere length is variable and depends directly on blood filling and the expansion of the heart chambers. In the human heart, maximal force is generated with an initial sarcomere length of 2.2 micrometres, a length rarely exceeded in a normal heart. Initial lengths larger or smaller than this optimal value will decrease the force the muscle can achieve. For longer sarcomere lengths, this is due to there being less overlap of the thin and thick filaments; for shorter sarcomere lengths, the cause is the decreased sensitivity of the myofilaments to calcium.
An increase in the filling of the ventricle increases the load experienced by each cardiac muscle cell, stretching their sarcomeres toward their optimal length. The stretching sarcomeres augment cardiac muscle contraction by increasing the calcium sensitivity of the myofibrils, causing a greater number of actin-myosin cross-bridges to form within the muscle. Specifically, the sensitivity of troponin for binding Ca2+ increases, and there is an increased release of Ca2+ from the sarcoplasmic reticulum.
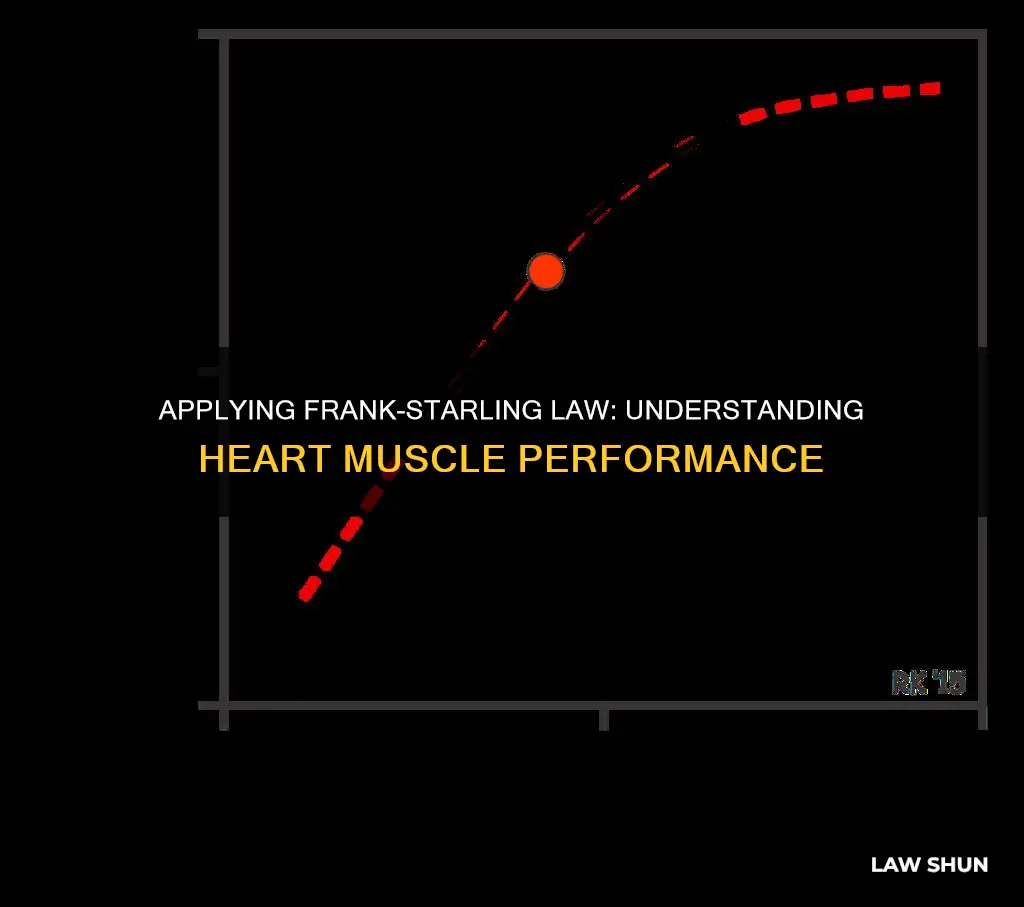
The Frank-Starling law is particularly relevant to the function of the left ventricle. The left ventricular performance (Frank-Starling) curves relate preload, measured as left ventricular end-diastolic volume (EDV) or pressure, to cardiac performance, measured as ventricular stroke volume or cardiac output. On the curve of a normally functioning heart, cardiac performance increases continuously as preload increases. During states of increased left ventricular contractility, for example, due to norepinephrine infusion, there is a greater cardiac performance for a given preload. This is represented graphically as an upward shift of the normal curve.
During states of decreased left ventricular contractility associated with systolic heart failure, there is decreased cardiac performance for a given preload compared to the normal curve. This is represented by a downward shift of the normal curve. Decreased contractility can also result from a loss of myocardium, as with myocardial infarction, beta-blockers (acutely), non-dihydropyridine Ca++ channel blockers, and dilated cardiomyopathy.
The Frank-Starling mechanism plays a role in the compensation of systolic heart failure, buffering the fall in cardiac output to help preserve sufficient blood pressure to perfuse the vital organs. Heart failure caused by the impaired contractile function of the left ventricle causes a downward shift of the left ventricular performance curve. At any given preload, the stroke volume will be decreased compared to normal. This reduced stroke volume leads to incomplete left ventricular emptying. Consequently, the volume of blood that accumulates in the left ventricle during diastole is greater than normal. The amplified residual volume increases the stretch of the myocardial fibres and induces a greater stroke volume with the next contraction via the Frank-Starling mechanism. This allows for better emptying of the enlarged left ventricle and preserves cardiac output.
Illinois Pharmacy Law Exam: Application Process Guide
You may want to see also

The Frank-Starling law and the role of calcium sensitivity
The Frank-Starling Law describes the relationship between the force of contraction and the initial length of muscle cells. The law states that the stroke volume of the left ventricle will increase as the left ventricular volume increases due to the myocyte stretch causing a more forceful systolic contraction. This is also known as the Frank-Starling mechanism, which describes the ability of the cardiac muscle to increase contractility in response to stretch or tension.
The Frank-Starling relationship is based on the link between the initial length of myocardial fibres and the force generated by contraction. There is an optimal length between sarcomeres at which the tension in the muscle fibre is greatest, resulting in the greatest force of contraction. The Frank-Starling mechanism is the observation that ventricular output increases as preload (end-diastolic pressure) increases.
Calcium sensitivity plays a role in the Frank-Starling Law. Length-dependent activation increases the formation of thick-filament strongly-bound cross-bridges on actin and imposes structural-mechanical alterations on the thin-filament with greater than normal bound Ca2+. Stretch-induced effects, rather than changes in filament spacing, appear to be primarily involved in the regulation of length-dependent activation.
The giant elastic protein titin may serve as a length-dependent mechanosensor. Titin is a huge protein that extends from the Z-disc to the M-band. Its N-terminal region interacts with actin in the Z-disc. The I-band region of titin is extensible and consists of three elastic components that act as a spring. In the A-band, titin is relatively inextensible because it interacts with the thick-filament proteins.
Titin-based passive tension is a factor in the Frank-Starling mechanism of the heart, by increasing length-dependent activation through an increase in calcium sensitivity at long sarcomere length. The N2B KO mouse model, in which titin-based passive tension is elevated, shows that titin-based passive tension is positively correlated with length-dependent activation.
In summary, the Frank-Starling Law describes the relationship between the force of contraction and the initial length of muscle cells. Calcium sensitivity plays a role in the Frank-Starling Law, with titin-based passive tension increasing calcium sensitivity at long sarcomere length.
Thermodynamics and DND: A Lawful Game?
You may want to see also

The Frank-Starling law and the impact on cardiac output
The Frank-Starling Law, also known as the Frank-Starling mechanism, describes the relationship between the stroke volume of the heart and the end-diastolic volume. The law states that the stroke volume of the heart increases in response to an increase in the volume of blood in the ventricles, before contraction (the end-diastolic volume), when all other factors remain constant.
The Frank-Starling mechanism allows the cardiac output to be synchronized with the venous return, arterial blood supply, and humoral length, without depending on external regulation to make alterations. The mechanism is of functional importance because it serves to adapt left ventricular output to right ventricular output.
The Frank-Starling mechanism occurs due to the length-tension relationship observed in striated muscle, including skeletal muscles, arthropod muscle, and cardiac muscle. As striated muscle is stretched, active tension is created by altering the overlap of thick and thin filaments. The greatest isometric active tension is developed when a muscle is at its optimal length.
In the human heart, maximal force is generated with an initial sarcomere length of 2.2 micrometres, a length which is rarely exceeded in a normal heart. Initial lengths larger or smaller than this optimal value will decrease the force the muscle can achieve. For longer sarcomere lengths, this is the result of there being less overlap of the thin and thick filaments; for shorter sarcomere lengths, the cause is the decreased sensitivity of myofilaments to calcium.
An increase in the filling of the ventricle increases the load experienced by each cardiac muscle cell, stretching their sarcomeres toward their optimal length. The stretching sarcomeres augment cardiac muscle contraction by increasing the calcium sensitivity of the myofibrils, causing a greater number of actin-myosin cross-bridges to form within the muscle.
The Frank-Starling Law plays a role in the compensation of systolic heart failure, buffering the fall in cardiac output to help preserve sufficient blood pressure to perfuse the vital organs. Heart failure caused by the impaired contractile function of the left ventricle causes a downward shift of the left ventricular performance curve. At any given preload, the stroke volume will be decreased as compared to normal. This reduced stroke volume leads to incomplete left ventricular emptying. Consequently, the volume of blood that accumulates in the left ventricle during diastole is greater than normal. The amplified residual volume increases the stretch of the myocardial fibres and induces a greater stroke volume with the next contraction via the Frank-Starling mechanism. This allows for better emptying of the enlarged left ventricle and preserves cardiac output.
Stark Law in Tennessee: Does It Apply to Nurse Practitioners?
You may want to see also
Frequently asked questions
The Frank-Starling Law, also known as Starling's Law, describes the







