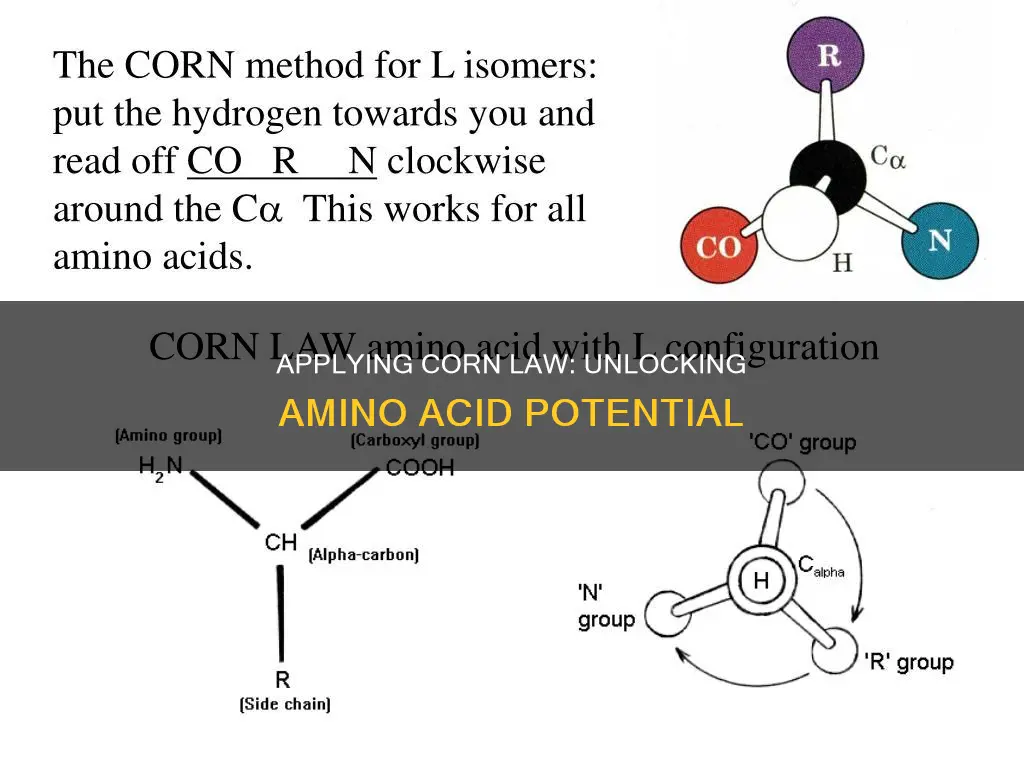
The CORN rule is used to distinguish enantiomers from each other and determine whether an amino acid is an L-isomer or a D-isomer. All amino acids have the same core but differ in their side chains. There are four different groups attached to the α-carbon, making α-amino acids chiral. These groups are the carboxylic acid group (-COO-), amino group (-NH2), a hydrogen atom, and a distinctive R group (side chain representation). The CORN acronym is derived from the first letters of the first three groups and the R group. If the carboxyl group is followed by the R group and the amino group in a clockwise direction, the amino acid is an L-isomer. If the movement is anticlockwise, the amino acid is a D-isomer.
| Characteristics | Values |
|---|---|
| What the CORN law is used for | To distinguish enantiomers from each other and determine whether an amino acid is a L-isomer or D-isomer |
| What CORN is an acronym for | -COOH, the -R and -NH2 groups |
| How to identify an L-isomer | If the carboxyl group is followed by the R group and the amino group in a clockwise direction |
| How to identify a D-isomer | If the carboxyl group is followed by the R group and the amino group in an anticlockwise direction |
| What the "corny rule" is | A hint to identify the natural type of amino acid |
What You'll Learn
- The CORN rule is used to distinguish enantiomers from each other
- Amino acids have a core and differ due to their side chain
- All amino acids have four different groups attached to their α-carbon
- The CORN rule determines whether an amino acid is an L-isomer or a D-isomer
- The CORN rule is a mnemonic to remember the arrangement of groups attached to the α-carbon

The CORN rule is used to distinguish enantiomers from each other
The CORN rule is used to determine whether an amino acid is an L-isomer or a D-isomer. All amino acids have the same core but differ due to their side chain. There are four different groups attached to α-carbon, making α-amino acids chiral. These groups are: a carboxylic acid group (-COOH), an amino group (-NH2), a hydrogen atom (-H) and a distinctive R group (side chain).
The CORN acronym stands for -COOH, -R and -NH2 groups. If the arrangement of these groups around the chiral centre carbon atom is counter-clockwise, with the hydrogen atom away from the viewer, then it is the L form. If the arrangement is clockwise, it is the D form. The L form is the usual one found in natural proteins.
To visualise this, you can use your left hand and wrap your fingers around the direction of CORN (-CO, -R, and -N groups in order). If your thumb points toward the direction of the hydrogen atom, then it is L-chiral. If you use your right hand, it is D-chiral.
Landlord Law: Home Shares and Legal Complications
You may want to see also

Amino acids have a core and differ due to their side chain
Amino acids are organic compounds that can exist in different forms depending on their environment. They are the fundamental building blocks of proteins and nitrogenous backbones for compounds such as neurotransmitters and hormones.
All amino acids have the same core and differ due to their side chain, also known as the R group. The R group varies and differentiates one amino acid from another. The core structure of an amino acid includes an amino functional group (-NH2) and a carboxylic acid functional group (-COOH). The term amino acid is short for α-amino [alpha-amino] carboxylic acid. Each molecule contains a central carbon (C) atom, called the α-carbon, to which both an amino and a carboxyl group are attached. The remaining two bonds of the α-carbon atom are generally occupied by a hydrogen (H) atom and the R group.
The side chain functional groups are specifically contributed by coordinating with metal ion centers to construct metal-organic frameworks (MOFs). The side chain is what gives amino acids a wide variety of structures and properties. There may be several carbon atoms between the amino and carboxyl groups. The first of them is called the α-atom, and if the side chain is bound to this atom, then the compound is called an α-amino acid.
The CORN law is the rule that allows us to distinguish enantiomers from each other. In other words, it determines whether the amino acid is an L-isomer or a D-isomer. The L or D chirality of amino acids is determined by the CORN rule: an amino acid is L-chiral (D-chiral) if by wrapping your left hand (right hand) fingers around the direction of CORN, your thumb points toward the direction of the hydrogen atom.
Open Meetings Law: Does It Apply in Kansas?
You may want to see also

All amino acids have four different groups attached to their α-carbon
Amino acids are organic compounds that are made up of a central carbon atom, also known as the alpha (α) carbon, bonded to an amino group, a carboxyl group, a hydrogen atom, and a side chain. The α-carbon is asymmetric, meaning that it is attached to four different substituents or groups. These four groups are:
- Carboxyl group (-COOH)
- Amino group (-NH2)
- Hydrogen atom (-H)
- R group or side chain, which varies depending on the specific amino acid
The R group is also known as the variable group or side chain and is unique to each amino acid. It is this group that determines the chemical nature and characteristics of the amino acid, including its size, polarity, and pH. The R group, along with the other three groups attached to the α-carbon, gives amino acids their chiral property. The L or D chirality of amino acids can be determined using the CORN rule, which is derived from the first letters of the four groups: Carboxyl (-CO), R group, and Amino (-NH2) group.
The structure of amino acids, with the four groups attached to the α-carbon, is essential for their function and role in proteins. Proteins are polymers of amino acids arranged in a linear sequence. The specific sequence and number of amino acids determine the protein's shape, size, and function. Each amino acid is attached to another through a peptide bond, formed by a dehydration synthesis reaction. This process results in the release of a molecule of water and the formation of a polypeptide chain.
The human body requires a variety of amino acids, including essential amino acids that cannot be produced by the body and must be obtained from the diet. The content and composition of amino acids in food proteins directly affect their nutritional value. Evaluating the amino acid profiles and compositions is crucial for understanding the nutritional value and quality of food proteins and for developing dietary nutrition guidelines.
Labor Law and Waitresses: What's the Verdict?
You may want to see also

The CORN rule determines whether an amino acid is an L-isomer or a D-isomer
The CORN rule is a method used to determine whether an amino acid is an L-isomer or a D-isomer. It is a commonly used mnemonic that differentiates enantiomers from each other.
All amino acids have the same core structure but differ in their side chains. There are four different groups attached to the α-carbon, making α-amino acids chiral. These groups are: a carboxylic acid group (-COO-), an amino group (-NH2), a hydrogen atom, and a distinctive R group (side chain).
The CORN rule is derived from the first letters of the chemical symbols of the first three groups mentioned above: -COOH (the carboxyl group), -R (the distinctive R group), and -NH2 (the amino group).
To apply the CORN rule, one must imagine looking along the Hydrogen-α Carbon bond of an amino acid. Starting at the carboxyl group, if you move your eyes in a clockwise direction and see the groups in the following order: -COOH, followed by -R, and then -NH2, then the amino acid is an L-isomer. If the movement is in the anticlockwise direction, the amino acid is a D-isomer.
The L- or D-chirality of amino acids can also be determined by wrapping your left hand (for L) or right hand (for D) around the direction of CORN (-CO, -R, and -N groups in order) and checking if your thumb points toward the direction of the hydrogen atom.
It is important to note that only one of the twenty naturally occurring amino acids is not in the L-form, and that is glycine. This exception occurs because the side chain group of glycine is a hydrogen atom, which results in the molecule being achiral or non-chiral.
The Castle Law: Front Yard Protection?
You may want to see also

The CORN rule is a mnemonic to remember the arrangement of groups attached to the α-carbon
The CORN rule is a mnemonic to help remember the arrangement of groups attached to the α-carbon. It is used to distinguish enantiomers from each other, determining whether an amino acid is an L-isomer or a D-isomer.
The four groups attached to the α-carbon are the carboxylic acid group (-COO-), amino group (-NH2), a hydrogen atom, and a distinctive R group (a representation of the side chain). The CORN acronym refers to -COOH, -R, and -NH2.
If the carboxyl group is followed by the R group and the amino group in a clockwise direction, the amino acid is an L-isomer. If the movement is in the anticlockwise direction, the amino acid is a D-isomer. This is known as the d-l convention and can be used with alpha amino acids.
The rule is as follows: imagine viewing the molecule along the H-C bond between the hydrogen and the asymmetric carbon atom. If the clockwise order of the other three groups is -COOH, -R, -NH2, the amino acid belongs to the d-series; otherwise, it belongs to the l-series.
The CORN rule is a useful mnemonic to determine the arrangement of groups attached to the α-carbon and, subsequently, whether an amino acid is an L- or D-isomer.
Lemon Law: Does It Expire After 18 Months?
You may want to see also
Frequently asked questions
The CORN law is a rule that helps distinguish enantiomers from each other. In other words, it determines whether an amino acid is an L-isomer or a D-isomer.
All amino acids have the same core and differ due to their side chain. There are four different groups attached to the α-carbon, making α-amino acids chiral. These groups are: a carboxylic acid group (-COO-), an amino group (-NH2), a hydrogen atom, and a distinctive R group (side chain representation). The CORN is an acronym for -COOH, the -R, and -NH2 groups. If the carboxyl group is followed by the R group and the amino group in a clockwise direction, the amino acid is an L-isomer. If the movement is in the anticlockwise direction, the amino acid is a D-isomer.
The CORN rule is a mnemonic to remember the arrangement of groups attached to the α-carbon. Imagine looking along the H-Cα bond with the H atom closest to you. When read clockwise, the groups attached to the Cα spell the word CORN.







