The law of definite proportions, also known as Proust's Law, is a scientific law that states that a chemical compound always contains the same elements in the exact same proportions by mass. This means that the proportions of elements in a compound will always remain the same, regardless of the amount of the compound being made or the source of its creation. For example, water, or H20, is made up of two atoms of hydrogen and one atom of oxygen, resulting in a composition of 11% hydrogen and 89% oxygen. This law was first observed by French chemist Joseph Proust in 1799, and it laid the foundation for modern chemistry by disproving the idea that any combination of elements could create a substance.
| Characteristics | Values |
|---|---|
| Name | Law of Definite Proportions |
| Other Names | Law of Constant or Fixed Proportion, Proust's Law |
| Proposer | French chemist Joseph Proust |
| Year Proposed | 1799 |
| Definition | A chemical compound always contains the same elements in the exact same proportions by mass |
| Examples | Water (H2O), Carbon Dioxide, Salt (NaCl), Sulfuric Acid (H2SO4), Ammonia, Glucose (C6H12O6), Vinegar (C2H4O2) |
What You'll Learn
- The law applies to pure chemical compounds, irrespective of their source or method of preparation
- The compound's composition is fixed
- The law was given by French chemist Joseph Proust in 1799
- The law is also known as Proust's Law
- The law ensures that chemical compounds are created using the same proportions

The law applies to pure chemical compounds, irrespective of their source or method of preparation
The law of definite proportions, also known as Proust's Law, states that a given chemical compound will always contain its constituent elements in a fixed ratio by mass. This ratio remains the same, regardless of the source of the compound or how it was prepared. This means that the composition of a compound is intrinsic to that compound and is not influenced by external factors.
For example, water (H2O) always has an oxygen atom combined with two hydrogen atoms. The atomic mass of oxygen is 16, while hydrogen has an atomic mass of 1. This means that water is made up of 11% hydrogen and 89% oxygen. This proportion is fixed—changing the ratio of hydrogen to oxygen would result in a different compound.
The law of definite proportions applies to pure chemical compounds, regardless of their source or method of preparation. This means that the composition of a compound is always the same, even if it was created in different ways or from different starting materials. For example, the composition of water will always be 11% hydrogen and 89% oxygen, whether it was prepared in a laboratory or found in nature. Similarly, the composition of carbon dioxide (CO2), which consists of one carbon atom and two oxygen atoms, will always be the same, regardless of the reactions that produced it.
The law of definite proportions was first proposed by French chemist Joseph Proust in 1797. At the time, it was a novel idea and it contributed to the development of atomic theory, which explained matter as consisting of discrete atoms, with different types of atoms combining in fixed proportions to form compounds.
Applying to Law School: A Comprehensive Guide
You may want to see also

The compound's composition is fixed
The law of definite proportions, also known as Proust's Law, states that a chemical compound always contains the same elements in the exact same proportions by mass. This means that the composition of a compound is fixed, and it will always contain the same elements in the same ratio, regardless of its source or the method of preparation.
For example, water (H2O) always has two atoms of hydrogen and one atom of oxygen. The atomic mass of hydrogen is 1, and the atomic mass of oxygen is 16, so water is always composed of 11% hydrogen and 89% oxygen by mass. This ratio is always true, no matter the amount of water or how it was formed. If a different ratio of hydrogen and oxygen were combined, a different compound would be created.
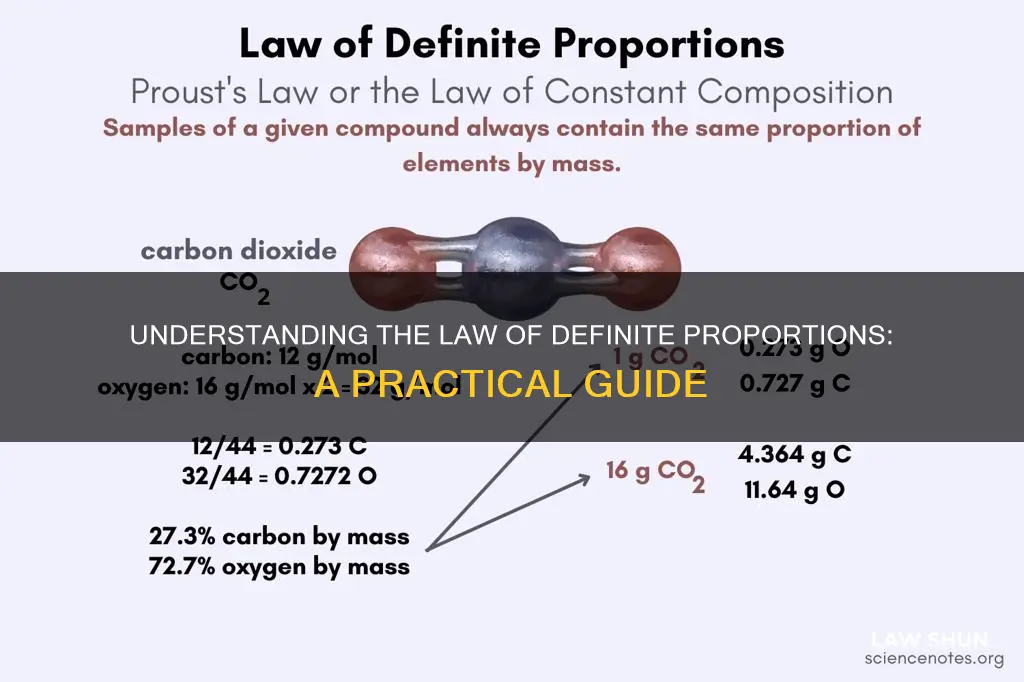
Another example is carbon dioxide (CO2), which always consists of one atom of carbon and two atoms of oxygen. The atomic mass of carbon is 12, and the atomic mass of oxygen is 16, so carbon dioxide always has a composition of 27.3% carbon and 72.7% oxygen by mass. This ratio is fixed, regardless of how the carbon dioxide was produced.
The law of definite proportions was first observed by French chemist Joseph Proust in 1799. It is based on Dalton's atomic theory, which states that atoms of an element are identical and have a fixed weight, so when they combine to form a compound, the resulting proportions of each element are also fixed.
California's Privacy Law: National Reach?
You may want to see also

The law was given by French chemist Joseph Proust in 1799
The law of definite proportions, also known as Proust's Law, was given by French chemist Joseph Proust in 1797 (or 1799 according to some sources). This law states that a chemical compound always contains its component elements in a fixed ratio by mass, and this ratio remains the same regardless of the amount of the compound or how it was prepared.
For example, water (H2O) is made up of two hydrogen atoms and one oxygen atom. The atomic mass of hydrogen is 1, and oxygen is 16, meaning that water is always made up of 11% hydrogen and 89% oxygen. The same principle applies to other compounds, such as salt (NaCl), sulfuric acid (H2SO4), and ammonia (NH3).
Proust's Law was a novel concept at the time, as some scientists believed that certain substances could be created by any combination of elements, rather than a definite proportion. The law contributed to the development of atomic theory, which was later promoted by John Dalton in 1805. Dalton's theory explained that matter consists of discrete atoms, with one type of atom per element, and that compounds are formed by combining different types of atoms in fixed proportions.
While the law of definite proportions forms the basis of stoichiometry, it is not universally true. There are non-stoichiometric compounds, such as the iron oxide wüstite, whose elemental composition can vary. However, Proust's measurements were not precise enough to detect such variations.
Kirchhoff's Voltage Law: Parallel Circuits Explained
You may want to see also

The law is also known as Proust's Law
The law of definite proportions is also known as Proust's Law, named after French chemist Joseph Proust (1754–1826), who first observed the law in 1797. It was, however, first published as a scientific proposal in 1794 by English chemist and theologian Joseph Priestly (1783–1804) and French chemist Antoine Lavoisier (1771–1794), based on their study of combustion.
Proust's Law states that a chemical compound always contains its elements in a fixed ratio by mass, regardless of the amount of the compound being made, where it came from, or how it was prepared. In other words, a given chemical compound will always contain the same elements in the exact same proportions by mass. For example, water (H2O) is made up of two hydrogen atoms and one oxygen atom. This means that water is made up of 11% hydrogen and 89% oxygen. The exact same proportion of hydrogen and oxygen must always be combined to create water.
The law of definite proportions is fundamental to the study of stoichiometry in chemistry. It is related to the law of multiple proportions and forms the basis, along with this law, of stoichiometry. The law also contributed to the atomic theory that John Dalton promoted from 1805, which explained matter as consisting of discrete atoms, with one type of atom per element, and that compounds were made of different types of atoms in fixed proportions.
Congress Insider Trading: Legal Loophole or Ethical Dilemma?
You may want to see also

The law ensures that chemical compounds are created using the same proportions
The law of definite proportions, also known as Proust's Law, is a fundamental principle in chemistry that ensures chemical compounds are consistently created with the same proportions of elements by mass. This law dictates that a chemical compound will always have a fixed ratio of its constituent elements, regardless of the amount of the compound being made or the source of its components.
For example, let's consider water, which is chemically represented as H2O. This means that water is made up of hydrogen and oxygen atoms. According to the law of definite proportions, the ratio of hydrogen to oxygen atoms in water is always 2:1. This means that any sample of water, regardless of its origin or preparation, will contain approximately 11% hydrogen and 89% oxygen by mass.
The law also applies to other compounds, such as carbon dioxide (CO2). Carbon dioxide is composed of one carbon atom and two oxygen atoms, resulting in a fixed ratio of 1:2. This knowledge is essential for understanding and predicting the outcomes of various chemical processes, such as combustion.
The discovery of the law of definite proportions was groundbreaking, as it challenged the prevailing belief that any combination of elements could form a substance. This law contributed significantly to the development of atomic theory, which explains matter as consisting of discrete atoms with unique elemental compositions.
While the law of definite proportions is a cornerstone of chemistry, it is important to note that there are exceptions. Some compounds, known as non-stoichiometric compounds, can exhibit variations in their elemental compositions. However, these exceptions do not diminish the significance and applicability of the law in understanding and predicting chemical reactions.
Maritime Law: Rivers and Their Legal Jurisdiction
You may want to see also







