The process of breathing is governed by Boyle's law, which states that the pressure of a fixed mass of gas is inversely proportional to its volume, given that its temperature is kept constant. In other words, an increase in volume leads to a decrease in pressure, and vice versa. This law helps us understand the relationship between pressure and volume in the lungs during respiration. When we breathe in, our chest expands, increasing the volume of the lungs and causing the pressure inside them to decrease. As a result, air rushes in from the atmosphere to fill the lungs. Conversely, when we breathe out, our chest contracts, reducing the volume of air in the lungs and increasing the pressure, which forces the air out.
| Characteristics | Values |
|---|---|
| Name of the Law | Boyle's Law |
| Discovery | Discovered by Robert Boyle in 1662 |
| First Notation | Richard Towneley and Henry Power |
| Relation | The volume of a gas and pressure are inversely proportional at a given temperature |
| Application in Breathing | As the lungs expand, the volume inside the lungs increases and the pressure inside decreases, allowing air to move inside the lungs from the outside |
What You'll Learn

Boyle's Law
The law is particularly relevant in understanding the process of breathing. When we inhale, our chest cavity expands, leading to an increase in volume. According to Boyle's Law, this increase in volume results in a slight decrease in pressure within our lungs compared to the pressure of the air outside our body. As a result, air rushes in to fill our lungs due to this pressure difference. Conversely, when we exhale, our chest cavity contracts, reducing the volume and increasing the pressure inside our lungs. This elevated pressure then forces the air out.
The application of Boyle's Law in respiration is not limited to humans. It also explains how changes in depth impact the gas volume and pressure experienced by scuba divers and deep-sea fish. For instance, when a scuba diver ascends rapidly from a deep zone to the water's surface, the decrease in pressure causes the gas molecules in their body to expand. These expanding gas bubbles can be incredibly harmful, even fatal, to the diver's organs.
Coulomb's Law: Understanding IMS Interactions
You may want to see also

Inspiration
As the diaphragm contracts, its dome shape becomes flatter and shallower, increasing the volume of the thoracic cavity. The external intercostal muscles elevate the ribs and sternum, also increasing the volume of the thoracic cavity. This increase in volume causes a slight drop in pressure, allowing air at atmospheric pressure to rush in and fill the lungs.
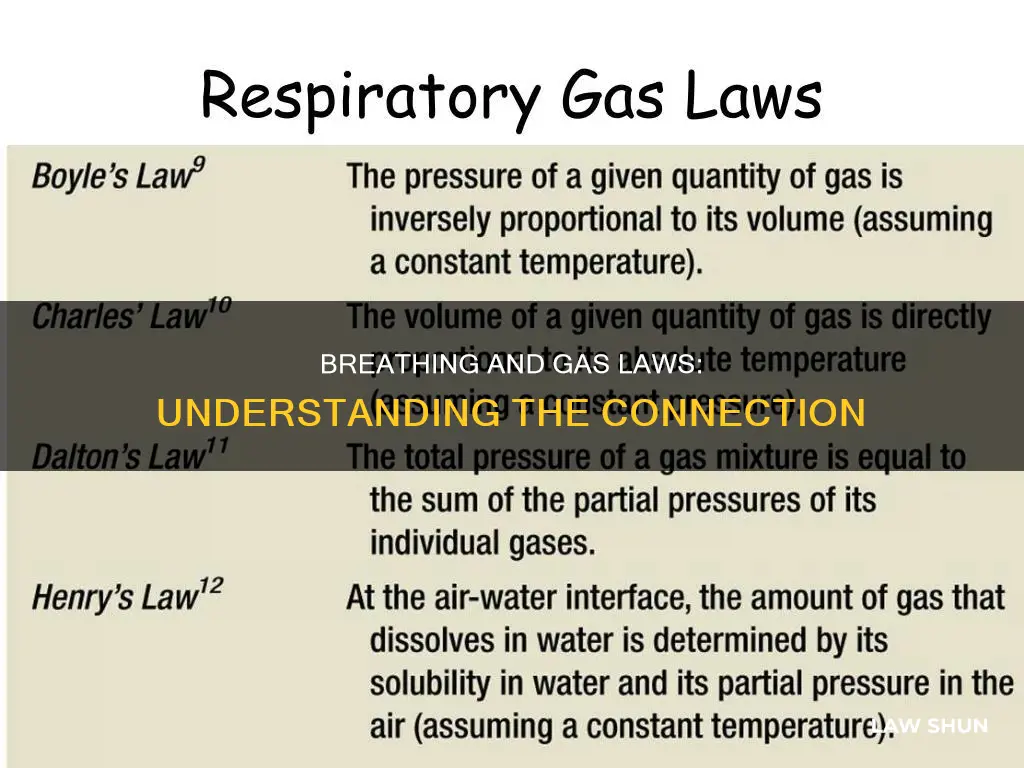
Boyle's Law describes the relationship between pressure and volume in a fixed mass of gas, stating that as volume increases, pressure decreases, and vice versa. This law applies to the process of inspiration, as the increase in volume of the thoracic cavity during inhalation causes a decrease in pressure, allowing air to be pushed into the lungs.
The formula for Boyle's Law is PV = K, where P is pressure, V is volume, and K is a constant. This can also be written as P1V1 = P2V2, indicating that the product of pressure and volume remains constant.
It is important to note that the lungs do not always follow Boyle's Law. At low and high volumes, the lung tissue's ability to expand or its elasticity decreases, resulting in low compliance. However, during normal breathing, the pressure inside the thoracic cavity changes only slightly, allowing for the inhalation and exhalation of air.
Criminal Law in Aviation: Application and Enforcement
You may want to see also

Expiration
During expiration, the diaphragm and external intercostal muscles relax. The diaphragm is a dome-shaped muscle that sits below the lungs. When it contracts, it increases the volume of the thoracic cavity, allowing the lungs to expand. When it relaxes, as in expiration, the diaphragm resumes its dome shape, reducing the volume of the thoracic cavity and increasing the pressure inside. This forces the air out of the lungs.
The internal and external intercostal muscles control the movement of the rib cage, with the external intercostal muscles also playing a role in expanding the lungs during inhalation. The contraction of these muscles during inhalation elevates the ribs and sternum, increasing the volume of the thoracic cavity. During exhalation, these muscles relax, allowing the ribs and sternum to lower and the volume of the thoracic cavity to decrease.
Boyle's Law describes the relationship between the pressure and volume of a gas, stating that if the volume increases, the pressure must decrease, and vice versa. This law applies to the process of breathing, with the volume of the lungs and thoracic cavity changing during inhalation and exhalation, resulting in changes in air pressure that allow air to move in and out of the lungs.
Understanding FMLA: Father-in-Law Coverage Explained
You may want to see also

Lung Compliance
The compliance of a system is defined as the change in volume that occurs per unit change in the system's pressure. In simpler terms, it refers to the ease with which an elastic structure stretches. In the context of the lungs, it measures the extent to which the lungs expand for each unit increase in trans-pulmonary pressure.
There are two types of lung compliance: static and dynamic. Static compliance refers to the measurement of pulmonary compliance at a fixed volume with no airflow and fully relaxed muscles. Dynamic compliance, on the other hand, refers to the continuous measurement of pulmonary compliance, calculated at each point to reflect schematic changes during rhythmic breathing. Dynamic compliance takes into account both elastic resistance and airway resistance.
The importance of lung compliance is evident in its application in mechanical ventilation for patients in intensive care or operating room environments. It is also crucial in understanding various pulmonary pathologies and guiding therapy.
Factors that affect lung compliance include the elastic properties of the lung tissue and surface tension. The elastic properties are determined by the collagen and elastin fibres within the lung parenchyma. The flexibility of these fibres is vital for lung compliance. Additionally, the surface tension of the alveolar lining also plays a role in lung compliance. The surface tension is influenced by the presence of surfactants, which are secreted by type II alveolar epithelial cells. Surfactants reduce surface tension, thereby increasing lung compliance.
Changes in lung compliance can indicate issues within the lungs and are often associated with injuries, illnesses, or impairments. For example, decreased compliance may be indicative of restrictive lung diseases, while increased compliance may be related to degenerative lung tissue diseases.
Community Property Law: Same-Sex Couples' Entitlement
You may want to see also

Gas Exchange
During inhalation, the diaphragm contracts, causing the thoracic cavity to expand. This contraction increases the volume of the lungs, leading to a slight drop in pressure. As a result, the pressure of the outside air becomes higher than the pressure inside the thoracic cavity, causing the outside air to rush in and fill the lungs.
When exhaling, the diaphragm relaxes, and the elastic fibres in the lung tissue cause the lungs to return to their original volume. This reduction in volume leads to an increase in pressure, forcing the air out of the lungs.
Boyle's law also applies in various other contexts, such as when using a medical syringe or when SCUBA divers descend and ascend to different depths. In the case of a syringe, pulling back on the plunger increases the volume in the cylinder, leading to a decrease in pressure, which then draws liquid into the cylinder to balance the pressure. For divers, as they descend, the pressure on their lungs increases, causing the air volume inside the lungs to decrease according to Boyle's law. As they ascend, the pressure decreases, resulting in an increase in air volume.
While Charles's law and Dalton's law are also relevant to respiration, they are less applicable than Boyle's law. Charles's law, which states that pressure increases as temperature increases, is less relevant as body temperature does not fluctuate significantly. On the other hand, Dalton's law, which states that each gas in a solution exerts its own pressure based on its concentration, is highly relevant as the air we breathe is made up of different gases, primarily nitrogen and oxygen.
HIPAA Laws: Do They Apply to Dentists?
You may want to see also
Frequently asked questions
Boyle's Law states that the pressure of a fixed mass of gas is inversely proportional to its volume, if its temperature is kept constant. In other words, when the pressure increases, the volume decreases, and vice versa.
During inhalation, our chest expands, increasing the volume of the lungs. This increase in volume causes a decrease in pressure inside the lungs, which is lower than the pressure outside. As a result, air is pushed into the lungs due to the pressure difference. When exhaling, the reverse happens.
Dalton's Law states that each of the gases in a gas solution exerts its own pressure based on its concentration in the solution. For example, the air we breathe is made up of nitrogen (78.6%) and oxygen (20.9%), and each of these gases exerts a different partial pressure.
Charles's Law is the least applicable of the three basic gas laws to respiration as it relates to temperature change, and body temperature rarely changes by much.