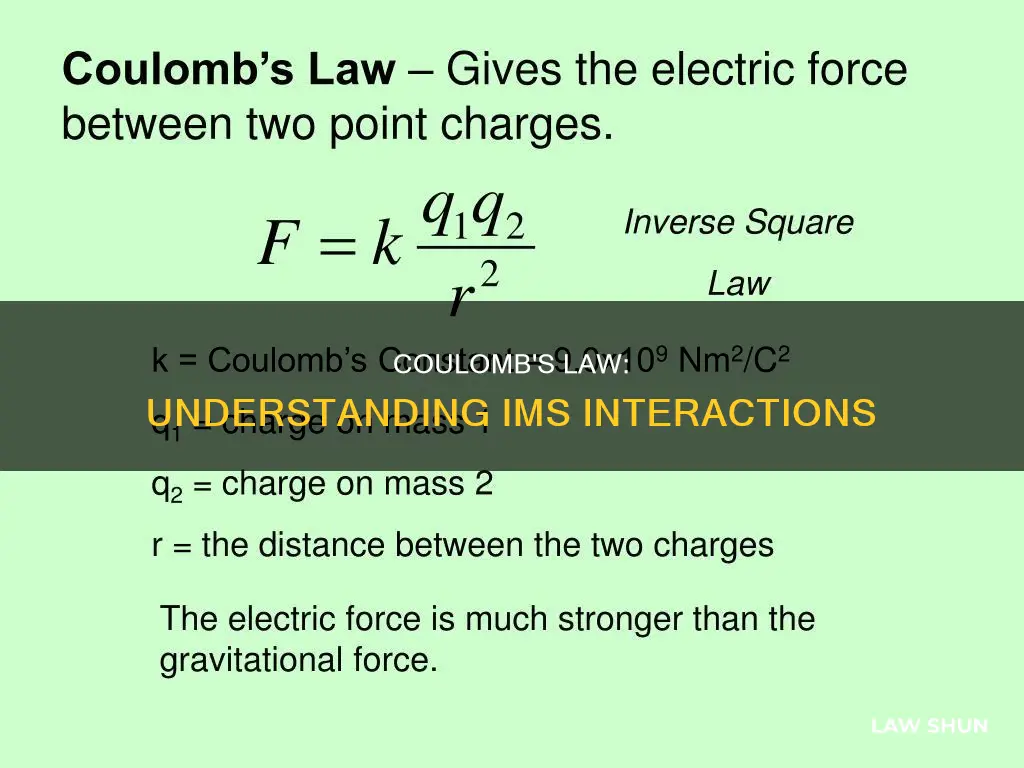
Coulomb's law, an experimental law of physics, calculates the force between two electrically charged particles at rest. It states that the force between two charged bodies is directly proportional to the product of their charges and inversely proportional to the square of the distance between them. This law is essential to the development of the theory of electromagnetism.
IMS, or ion mobility spectrometry, is a technique used to separate and identify ionised molecules based on their mobility in a carrier gas. Coulomb's law is applicable to IMS as it helps determine the electrostatic force between charged particles, which is crucial for understanding the behaviour of ions in an electric field. By applying Coulomb's law, scientists can calculate the force between ions and the strength of the electric field, enabling them to analyse and identify the ions in a sample.
| Characteristics | Values |
|---|---|
| Definition | A mathematical concept that defines the electric force between charged objects |
| Formula | F = k * (Q1 * Q2) / d^2 |
| F = force | |
| Q1 and Q2 = charges | |
| d = distance | |
| k = Coulomb's constant | |
| Discovery | First observed by Greek philosopher Thales of Miletus in 600 BC |
| Formalised by French physicist Charles-Augustin de Coulomb in 1785 | |
| Application | Used to calculate the distance and force between two charges |
| Used to calculate the electric field | |
| Used to arrange charges in stable equilibrium | |
| Limitations | Only applicable to point charges at rest |
| Only applicable in situations where the inverse square law is followed | |
| Not applicable to charges with arbitrary shapes |
What You'll Learn
- The force between charged bodies is a non-contact force
- Coulomb's Law can be used to calculate the distance and force between two charges
- The electrostatic force is directly proportional to the product of the charges
- Coulomb's Law is only applicable to point charges at rest
- The force between two charges depends on the nature of the intervening medium

The force between charged bodies is a non-contact force
Coulomb's Law, an experimental law of physics, calculates the amount of force between two electrically charged particles at rest. The law states that the magnitude or absolute value of the attractive or repulsive electrostatic force between two point charges is directly proportional to the product of the magnitudes of their charges and inversely proportional to the square of the distance between them.
The force between charged bodies is determined by the charges and the distance between them. The force is stronger when the magnitude of the charge is higher, and weaker when the distance between the charges is larger.
Coulomb's Law can be used to calculate the distance and force between two charges. It can also be used to calculate the force on one point due to the presence of several points, known as the Theorem of Superposition.
Hunting Laws in California: BLM Land Rules Explained
You may want to see also

Coulomb's Law can be used to calculate the distance and force between two charges
Coulomb's Law, also known as Coulomb's inverse-square law, can be used to calculate the force and distance between two charges. The law states that the force between two electrically charged particles is directly proportional to the product of their charges and inversely proportional to the square of the distance between them.
The formula for Coulomb's Law is:
F = ke * q1 * q2 / r^2
Where:
- F is the force between the charges
- Ke is the Coulomb constant
- Q1 and q2 are the magnitudes of the two charges
- R is the distance between the charges
By inputting the magnitudes of the two charges and the distance between them into this formula, the force between the charges can be calculated.
Additionally, if the magnitudes of the two charges and the force between them are known, the distance between the charges can be calculated using the following formula:
R = sqrt(ke * q1 * q2 / F)
This formula can be used to solve for the distance between the charges.
Coulomb's Law can also be used to calculate the charge of one of the objects if the charge, force, and distance between the objects are known. The formula for this is:
Q2 = F * r^2 / ke / q1
Overall, Coulomb's Law provides a useful framework for calculating the force, distance, and charge of objects with electrical charges.
Gas Laws: Building Construction and Safety
You may want to see also

The electrostatic force is directly proportional to the product of the charges
Coulomb's law, an experimental law of physics, calculates the amount of force between two electrically charged particles at rest. This electric force is known as the electrostatic force. The law states that the magnitude of the attractive or repulsive electrostatic force between two point charges is directly proportional to the product of the magnitudes of their charges.
The direction of the force is along the line joining the centres of the two charged particles. If the charges have the same sign, the force between them will be repulsive; if they have different signs, the force will be attractive.
Coulomb's law is similar to Newton's law of universal gravitation, but there are two important differences. Firstly, gravitational forces are always attractive, whereas electrostatic forces can be attractive or repulsive. Secondly, gravitational forces are much weaker than electrostatic forces.
Thermodynamics and DND: A Lawful Game?
You may want to see also

Coulomb's Law is only applicable to point charges at rest
Coulomb's law, an experimental law of physics, calculates the amount of force between two electrically charged particles at rest. It states that the magnitude of the attractive or repulsive electrostatic force between two point charges is directly proportional to the product of the magnitudes of their charges and inversely proportional to the square of the distance between them.
Coulomb's law is not valid for moving charges. This is because the information about the position of the charge (the field caused by the charge) can only travel at the speed of light. For moving charges, there is an extra factor introduced, which alters the force produced on the two objects. This extra part of the force is called the magnetic force.
Coulomb's law is a fundamental principle in physics that describes the electrostatic interaction between two charged particles. It is not universal, as it depends on the properties of the intervening medium. The electrostatic force is conventionally called the Coulomb force.
Lemon Law Loophole: ATVs and Washington's Law
You may want to see also

The force between two charges depends on the nature of the intervening medium
Coulomb's Law, an experimental law of physics, calculates the amount of force between two electrically charged particles at rest. The force between two charges depends on the nature of the intervening medium.
The force between charged bodies is not a contact force. It exists over a length, and all electrical interactions have a force embedded in them. The charges and the distance between the charged bodies determine the power and influence of the force. The force is directly proportional to the product of the charges and inversely proportional to the square of the distance between them. It acts along the line joining the two charges, considered to be point charges.
The force between two charges depends on the permittivity of the intervening medium. Permittivity is a property of the medium between the charges. The permittivity of a material is calculated by dividing the force between two charges in air by the force between the same charges in the medium at the same distance.
The force between two charges in a medium is given by the equation:
F = (1 / (4πεo)) x (q1 x q2) / d2
Where F is the force, q1 and q2 are the charges, d is the distance between the charges, and εo is the permittivity of free space.
The permittivity of free space or a vacuum is 1. The permittivity of other materials is greater than 1. For example, the permittivity of water is around 80.
The force between two charges in a medium can be different from the force between the same charges in a vacuum due to the polarisation of the medium by the electric field of the charges. This polarisation affects the strength of the electric field and, consequently, the force between the charges.
Coulomb's Law is not universal, as it depends on the properties of the intervening medium. It is only applicable to stationary point charges at rest.
Delaware Corporation Law: California Compliance and Considerations
You may want to see also