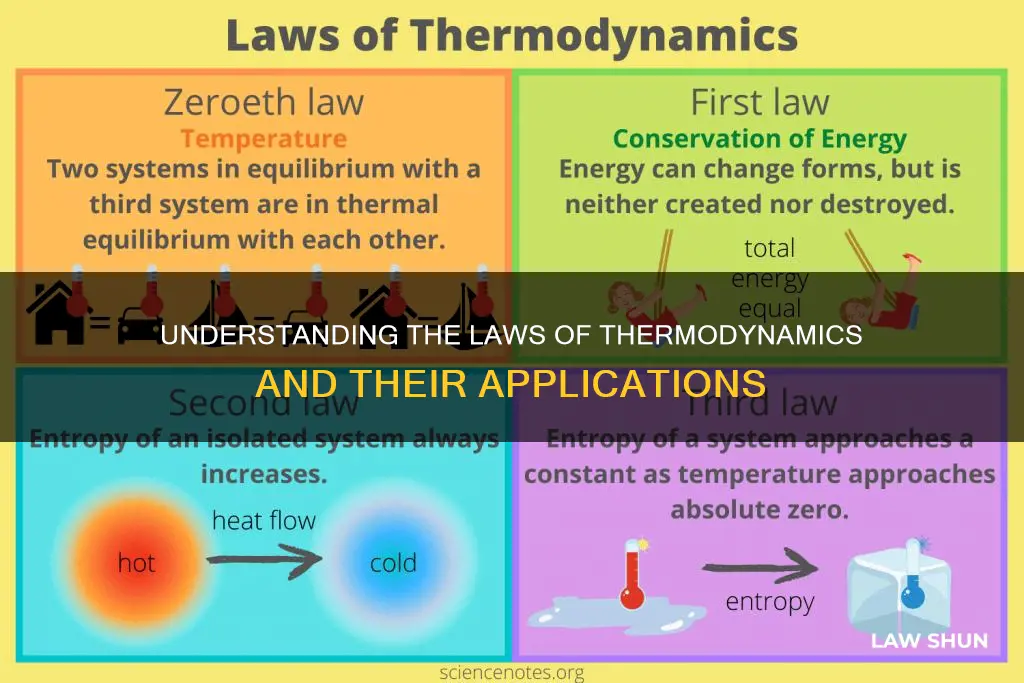
The laws of thermodynamics are a set of scientific laws that define a group of physical quantities, such as temperature, energy, and entropy, and govern the transfer of energy in and among all systems in the universe. Traditionally, there are three fundamental laws: the first law, the second law, and the third law. However, a fourth law, known as the zeroth law, was later added to provide a self-consistent definition of temperature. The first law of thermodynamics, also known as the law of conservation of energy, states that energy cannot be created or destroyed, only transformed from one form to another. The second law of thermodynamics states that the sum of the entropies of interacting thermodynamic systems never decreases, leading to an increase in disorder or randomness. The third law of thermodynamics focuses on the behaviour of systems as their temperature approaches absolute zero, stating that the system's entropy approaches a constant value. Meanwhile, the zeroth law defines thermal equilibrium and forms the basis for the definition of temperature. These laws have broad applicability, not only in thermodynamics but also in other natural sciences, providing fundamental insights into the workings of the universe.
| Characteristics | Values |
|---|---|
| First Law of Thermodynamics | Energy cannot be created or destroyed |
| The total amount of energy in the universe is constant | |
| Energy can be transferred or transformed | |
| Second Law of Thermodynamics | For a spontaneous process, the entropy of the universe increases |
| For a spontaneous process, ΔSuniverse > 0 | |
| For a spontaneous process, ΔSsystem + ΔSsurroundings > 0 | |
| The entropy of a closed system increases over time | |
| Heat does not flow spontaneously from a colder region to a hotter region | |
| Third Law of Thermodynamics | A perfect crystal at zero Kelvin has zero entropy |
| Zeroth Law of Thermodynamics | If two bodies are each in thermal equilibrium with a third body, they must also be in equilibrium with each other |
What You'll Learn
- The Zeroth Law of Thermodynamics states that if two bodies are in thermal equilibrium with a third body, they are in thermal equilibrium with each other
- The First Law of Thermodynamics, or the law of conservation of energy, states that energy can be converted from one form to another but cannot be created or destroyed
- The Second Law of Thermodynamics states that the entropy of an isolated system always increases
- The Third Law of Thermodynamics states that a system's entropy approaches a constant value as its temperature approaches absolute zero
- The Second Law of Thermodynamics also states that heat does not spontaneously pass from a colder body to a warmer body

The Zeroth Law of Thermodynamics states that if two bodies are in thermal equilibrium with a third body, they are in thermal equilibrium with each other
The Zeroth Law of Thermodynamics is one of the four principal laws of thermodynamics. It was established by Ralph H. Fowler in the 1930s, long after the first, second, and third laws were widely recognised.
The Zeroth Law states that if two bodies are in thermal equilibrium with a third body, they are in thermal equilibrium with each other. In other words, the three bodies are at the same temperature. This law is important for the mathematical formulation of thermodynamics and the definition of temperature.
The Zeroth Law defines thermal equilibrium and forms the basis for the definition of temperature. Two systems are said to be in thermal equilibrium if they are linked by a wall permeable only to heat, and they do not change over time. Thermal equilibrium means that when two bodies are brought into contact with each other and separated by a barrier that is permeable to heat, there will be no transfer of heat from one to the other.
The Zeroth Law is needed for the definition of temperature scales and justifies the use of practical thermometers. It establishes that temperature is a fundamental and measurable property. For a thermometer to be useful, it must be calibrated. This is done by observing how a certain characteristic of the body changes with temperature. The change in the characteristic may be taken as an indication of a change in temperature. The selected characteristic is known as a thermodynamic property.
The Zeroth Law also provides a foundation for temperature as an empirical parameter in thermodynamic systems and establishes the transitive relation between the temperatures of multiple bodies in thermal equilibrium.
Contract Law: Which Rules Apply?
You may want to see also

The First Law of Thermodynamics, or the law of conservation of energy, states that energy can be converted from one form to another but cannot be created or destroyed
The First Law of Thermodynamics, also known as the law of conservation of energy, is a fundamental principle in physics that describes the behaviour of energy in any given
Anti-Discrimination Laws: Global Reach of US Legislation?
You may want to see also

The Second Law of Thermodynamics states that the entropy of an isolated system always increases
The Second Law of Thermodynamics is a physical law based on the universal empirical observation of heat and energy interconversions. It establishes the concept of entropy as a physical property of a thermodynamic system. Entropy is a measure of the disorder of a system and is a difficult concept to grasp as it may seem abstract. However, we can see examples of entropy in our everyday lives. For instance, if a car tire is punctured, the air escapes and disperses in all directions. This dispersal of energy is what entropy measures.
The law can be formulated by the observation that the entropy of isolated systems left to spontaneous evolution cannot decrease, as they always tend towards a state of thermodynamic equilibrium where the entropy is highest at the given internal energy. This increase in the combined entropy of the system and its surroundings accounts for the irreversibility of natural processes, often referred to as the "arrow of time".
The Second Law of Thermodynamics can be applied to both reversible and irreversible processes. For a reversible or quasi-static, idealized process of energy transfer as heat to a closed thermodynamic system, an infinitesimal increment in the entropy of the system is defined as the infinitesimal transfer of heat to the system, divided by the common thermodynamic temperature of the system and its surroundings.
The Second Law determines whether a proposed physical or chemical process is forbidden or may occur spontaneously. For isolated systems, no energy is provided by the surroundings, and the Second Law requires that the entropy of the system alone must increase. Examples of spontaneous physical processes in isolated systems include heat transfer from a region of higher temperature to lower temperature, the conversion of mechanical energy to thermal energy, and the movement of a solute from a region of higher concentration to lower concentration.
However, for some non-isolated systems that can exchange energy with their surroundings, the surroundings may exchange enough heat or do sufficient work on the system so that the processes occur in the opposite direction. This is possible as long as the total entropy change of the system and its surroundings is positive, as required by the Second Law.
The Second Law is a statistical law, applying generally to macroscopic systems. It does not preclude small-scale variations in the direction of entropy over time. In fact, the Fluctuation Theorem states that as the length of time or the system size increases, the probability of a negative change in entropy decreases exponentially. So, on very small time scales, there is a real probability that fluctuations of entropy against the Second Law can exist.
Unraveling Prejudice's Role in Law and Justice
You may want to see also

The Third Law of Thermodynamics states that a system's entropy approaches a constant value as its temperature approaches absolute zero
The Third Law of Thermodynamics, developed by German chemist Walther Nernst between 1906 and 1912, states that a system's entropy approaches a constant value as its temperature approaches absolute zero. This means that as the temperature of a closed system gets closer to zero Kelvin (0 K), the disorder or randomness in the system, as measured by its entropy, remains stable.
Entropy, denoted by 'S', is a measure of the disorder or randomness in a closed system. It is directly related to the number of microstates accessible by the system. The microstate in which the energy of the system is at its minimum is called the ground state of the system.
At absolute zero, the system is in the ground state, with the minimum possible energy. The constant value of entropy at this point is called the residual entropy of the system. In a perfect crystal, where there is only one unique ground state, the entropy at absolute zero is exactly zero.
However, for systems without a well-defined order, such as non-crystalline solids or glasses, there may be some finite residual entropy even at absolute zero. This is because the system may become locked into a configuration with non-minimal energy or because the minimum energy state is not unique.
The Third Law provides an absolute reference point for determining the entropy of a system at any other temperature. By selecting the initial entropy value at absolute zero as zero (S0 = 0), the entropy of a closed system determined relative to this zero point is the absolute entropy of that system.
The Third Law has several formulations, including the Nernst statement, which concerns thermodynamic processes at fixed, low temperatures for condensed systems (liquids and solids), and the Einstein statement, which asserts that the entropy of any substance approaches a finite value as the temperature approaches absolute zero.
Space Law: Jurisdiction Beyond Earth's Boundaries
You may want to see also

The Second Law of Thermodynamics also states that heat does not spontaneously pass from a colder body to a warmer body
The laws of thermodynamics are a set of scientific laws that define a group of physical quantities, such as temperature, energy, and entropy, that characterise thermodynamic systems in thermodynamic equilibrium. The laws also establish relationships between various parameters for thermodynamic processes, such as thermodynamic work and heat. The Second Law of Thermodynamics is a physical law based on universal empirical observation concerning heat and energy interconversions.
The Second Law of Thermodynamics states that, in a natural thermodynamic process, the sum of the entropies of the interacting thermodynamic systems never decreases. This law establishes the concept of entropy as a physical property of a thermodynamic system. Entropy is a measure of disorder or chaos in a system. The Second Law dictates that entropy always increases over time, and spontaneous processes are those that occur without external influence, always converting order to disorder.
A common corollary of the Second Law is that heat does not spontaneously pass from a colder body to a warmer body. This statement, known as the Clausius statement, was formulated by Rudolf Clausius in the 1850s. It implies that heat can never pass from a colder to a warmer body without some other change occurring simultaneously. This is evident from ordinary experiences, such as refrigeration, where heat is transferred from cold to hot, but only when forced by an external agent or system.
The Second Law also indicates the irreversibility of natural processes and, in many cases, the tendency of natural processes to lead towards spatial homogeneity of matter and energy, especially temperature. It asserts that a natural process runs only in one direction and is not reversible. While the state of a natural system can be reversed, it cannot be fully reversed without increasing the entropy of the system's surroundings.
The Second Law provides criteria for spontaneous processes and helps determine whether a proposed physical or chemical process is forbidden. For example, it allows for a cup falling off a table and breaking but denies the reverse process of the cup fragments coming back together and jumping back onto the table.
Criminal Law: Modern Society's Legal Framework
You may want to see also
Frequently asked questions
The first law of thermodynamics, also known as the law of conservation of energy, states that energy can neither be created nor destroyed, but only changed from one form to another.
The second law of thermodynamics states that the entropy of an isolated system will always increase over time and that the entropy of the universe can never decrease.
The third law of thermodynamics states that the entropy of a system approaches a constant value as the temperature approaches absolute zero. In other words, as the temperature of a system gets closer to absolute zero, it becomes increasingly difficult to extract energy from it.
The zeroth law of thermodynamics states that if two bodies are in thermal equilibrium with a third body, then the first two bodies are also in thermal equilibrium with each other. This enables the use of thermometers to compare the temperatures of different objects.