Boyle's law, also known as Mariotte's law, is a fundamental principle in chemistry that describes the behaviour of gas at a constant temperature. The law was formulated by Robert Boyle in 1662 and states that the pressure exerted by a gas is inversely proportional to the volume it occupies, provided the temperature and amount of gas remain constant. However, Boyle's law has its limitations and does not apply in certain situations. This paragraph aims to introduce the topic of When does Boyle's law not apply? by exploring scenarios where deviations from the ideal gas behaviour occur and understanding the factors that influence the relationship between pressure and volume.
| Characteristics | Values |
|---|---|
| Temperature | Must be held constant |
| Pressure | Must be held constant |
| Volume | Must be held constant |
| Mass | Must be held constant |
| Quantity of gas | Must be held constant |
| Temperature and pressure | Must be low |
What You'll Learn

Boyle's Law at high pressure
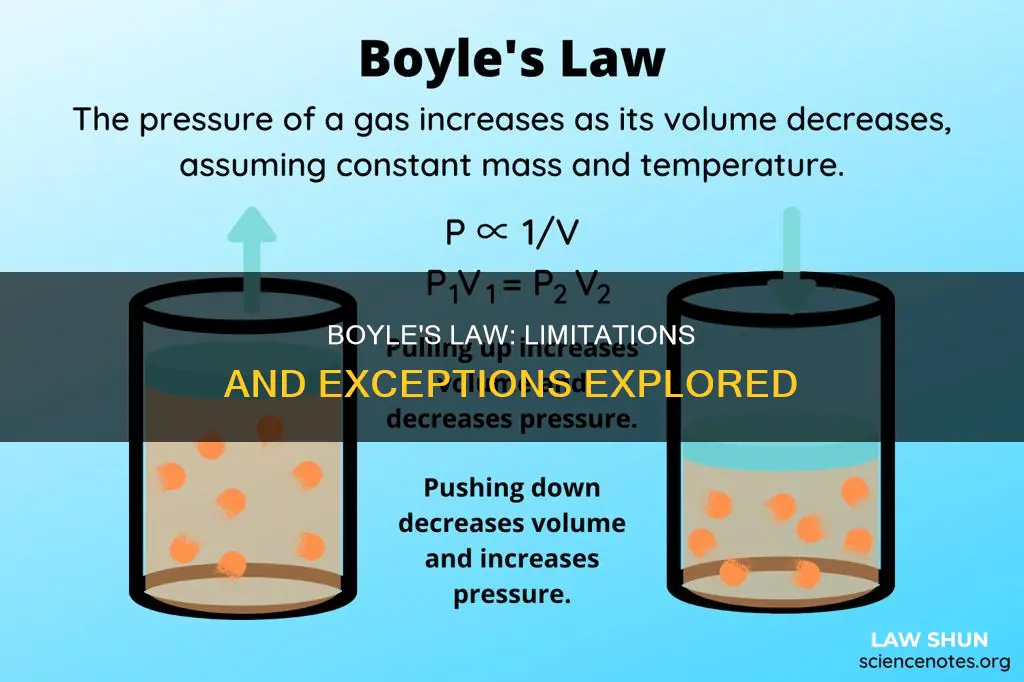
Boyle's Law, also known as the Boyle-Mariotte Law, is an empirical gas law that describes the relationship between the pressure exerted by a gas and the volume it occupies. The law states that, for a given mass of an ideal gas kept at a fixed temperature, pressure and volume are inversely proportional. This means that, if the temperature and the amount of gas remain constant, an increase in pressure will result in a decrease in volume, and vice versa.
The law was formulated by the Anglo-Irish chemist Robert Boyle in 1662 and can be expressed mathematically as:
P ∝ (1/V)
Or
P = k*(1/V) ⇒ PV = k
Where P is the pressure exerted by the gas, V is the volume occupied by it, and k is a constant.
Boyle's Law is based on experiments conducted by Boyle, who used a closed J-shaped tube and poured mercury from one side to force the air on the other side to contract under the pressure. By repeating this experiment with different amounts of mercury, he found that the pressure of a gas is inversely proportional to the volume it occupies under controlled conditions.
However, Boyle's Law has limitations and does not apply at high pressures. This is because, at high pressures, gases behave like ideal gases, and deviations from ideal gas behaviour become noticeable. Real gases obey Boyle's Law at sufficiently low pressures, but as pressure increases, the product PV generally decreases slightly, and the gas begins to depart from ideal behaviour.
To accurately describe the relationship between pressure and volume at high pressures, a more complex theory is needed, such as the real gas theory, which takes into account the compressibility factor.
Antitrust Laws: Conglomerate Mergers and Their Exemptions
You may want to see also

Real gases
Boyle's law, also known as Mariotte's law, is an empirical gas law that describes the relationship between the pressure exerted by a gas and the volume it occupies when its temperature and quantity are held constant. The law is expressed as:
PV = k
Where P is the pressure exerted by the gas, V is the volume occupied, and k is a constant.
While the law is useful for understanding the behaviour of ideal gases, it has limitations when applied to real gases. Real gases only obey Boyle's law at sufficiently low pressures. At higher pressures, the product of pressure and volume (PV) decreases slightly as the gas begins to deviate from ideal behaviour. This deviation is expressed as the compressibility factor.
The law was formulated based on experiments conducted by Robert Boyle in 1662, and independently discovered by French physicist Edme Mariotte in 1676 or 1679.
Homeland Law: Immigration Inclusions and Exemptions
You may want to see also

The discovery of Boyle's Law
Robert Boyle, an Anglo-Irish natural philosopher, chemist, physicist, alchemist and inventor, is known for formulating what we now call Boyle's Law. He is regarded as the first modern chemist and one of the pioneers of modern experimental scientific methods.
Boyle's Law, also referred to as the Boyle-Mariotte Law, describes the relationship between the pressure exerted by a gas and the volume it occupies. It states that the pressure and volume of a gas are inversely proportional to each other, as long as the temperature and the quantity of the gas remain constant.
The relationship between pressure and volume was first noted by Richard Towneley and Henry Power in the 17th century. Robert Boyle then confirmed their discovery through experiments and published the results in 1662. According to Robert Gunther and other authorities, it was Boyle's assistant, Robert Hooke, who built the experimental apparatus.
Boyle's Law is based on experiments with air, which he considered to be a fluid of particles at rest between small invisible springs. One of his experiments involved using a closed J-shaped tube and pouring mercury from one side to force the air on the other side to contract under the pressure. He repeated this experiment several times, using different amounts of mercury, and found that under controlled conditions, the pressure of a gas is inversely proportional to the volume occupied by it.
Boyle's Law was the first physical law to be expressed in the form of an equation describing the dependence of two variable quantities. The mathematical equation for Boyle's Law is: PV=k, where P denotes the pressure of the system, V denotes the volume of the gas, and k is a constant value representative of the temperature of the system and the amount of gas.
Lemon Law and Tires: What's the Deal?
You may want to see also

Application of Boyle's Law
Boyle's Law, also known as Mariotte's law, is an empirical gas law that describes the relationship between the pressure and volume of a confined gas. It was formulated by Anglo-Irish chemist and physicist Robert Boyle in 1662. The law states that the pressure exerted by a gas is inversely proportional to the volume occupied by it, as long as the temperature and the quantity of gas remain constant.
Mathematically, Boyle's Law can be stated as: PV = k, where P denotes the pressure of the system, V denotes the volume of the gas, and k is a constant value representative of the temperature of the system and amount of gas.
Spray Paint
Spray paint or aerosol spray is a classic example of Boyle's Law in action. A spray paint container usually contains two substances: the paint material itself and a compressed gas in a liquid state. The liquefied gas has a boiling point below room temperature, but it doesn't boil and turn into a gas because the container is sealed. When you press the sprayer, the gas starts to escape, causing the liquefied gas to expand and turn into gas. This gas then pushes the paint inside the container out through the sprayer nozzle.
Soda Bottle
Soda bottles or cans are another practical application of Boyle's Law. When you open a bottle of soda quickly, the gas escapes from everywhere in the form of foam, creating a mess. This happens because the soda is pumped into the bottle by passing it through carbon dioxide. When you open the bottle, you are reducing the pressure on the gas, causing its volume to expand. Opening the cap slowly and carefully allows the gas to escape quietly without creating a mess.
Diving
Diving in deep water also illustrates Boyle's Law. As a diver moves deeper underwater, the pressure increases, leading to a decrease in volume, and the diver's blood begins to absorb nitrogen gas. When the diver starts to ascend, the nitrogen gas molecules expand and return to their original volume. A slow ascent allows the nitrogen gas molecules to expand and return to normal without problems. However, if the diver ascends too quickly, the blood can turn into a foamy mess, causing the diver to bend over in severe pain and, in the worst cases, leading to instant death.
Breathing
Boyle's Law also applies to the human breathing system. When a person breathes in, their lung volume increases, and the pressure within decreases, drawing air into the lungs. During exhalation, the lung volume decreases, the pressure within increases, and air is forced out of the lungs.
HIPAA Laws and Minors: Privacy Rights Explained
You may want to see also

The mathematical equation for Boyle's Law
Boyle's law, also known as the Boyle-Mariotte law, is an empirical gas law that describes the relationship between the pressure and volume of a confined gas. It was formulated by Anglo-Irish physicist Robert Boyle in 1662.
PV = k
Where P denotes the pressure of the system, V denotes the volume of the gas, and k is a constant value representative of the temperature of the system and amount of gas.
This equation shows that the pressure and volume of a gas are inversely proportional to each other as long as the temperature and the quantity of gas remain constant. In other words, when the volume increases, the pressure decreases, and vice versa.
For example, if the volume is doubled, the pressure is halved, and if the volume is halved, the pressure is doubled.
Boyle's law can be used to predict the change in pressure when the volume of a gas is altered, as shown in the following equation:
P1V1 = P2V2
Where P1 and V1 represent the original pressure and volume, and P2 and V2 represent the new pressure and volume.
Boyle's law is a special case of the ideal gas law and is applicable to real gases at normal temperatures and low pressures. However, as temperature and pressure increase, gases deviate from the ideal gas law, and the relationship between pressure and volume can only be described using real gas theory.
US Law: Global Reach and Overseas Applicability
You may want to see also